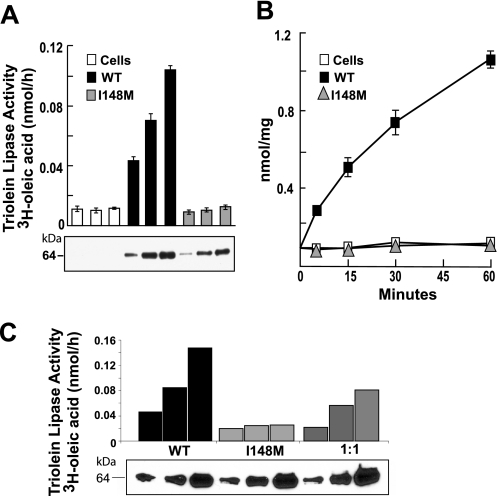
FIGURE 2.
Effect of I148M substitution on triglyceride hydrolysis in vitro. FLAG-tagged human wild type and mutant (I148M) PNPLA3 were partially purified from Sf9 cells using nickel affinity chromatography as described under “Experimental Procedures.” A, a total of 20, 40, or 80 μg of protein was incubated at 37 °C for 15 min with 3H-triolein emulsions (60 μm of [9,10-3H]triolein). Lipids were extracted with butanol and separated by TLC, and the free fatty acid bands were excised and quantitated by scintillation counting. B, emulsions of radiolabeled triolein were incubated with 40 μg of partially purified recombinant PNPLA3 for the times indicated. The free fatty acid release was quantitated as described in A. C, partially purified wild type PNPLA3, PNPLA3-I148M and a 1:1 mixture of wild type and mutant PNPLA3 were incubated with [3H]triolein emulsions, and free fatty acid release was measured as described in A. Proteins were examined by immunoblotting using an anti-FLAG epitope antibody (Sigma). Each experiment was repeated twice, and similar results were obtained. WT, wild type.