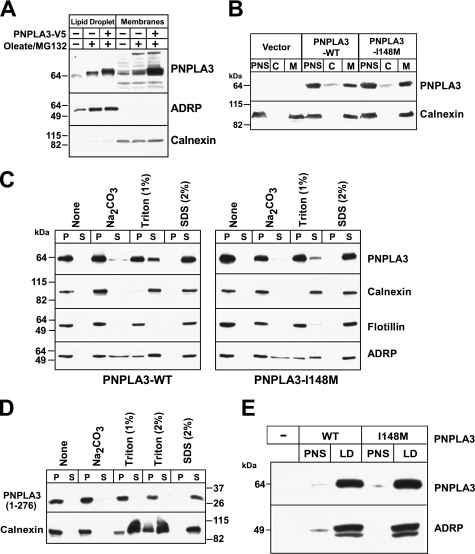
FIGURE 5.
Subcellular localization of PNPLA3 in cultured hepatoma (HuH-7) cells. A, HuH-7 cells were grown in the absence and presence of 400 μm oleate for 24 h and MG132 (2.5 μm) for 12 h. Membranes and lipid droplets were isolated by density gradient ultracentrifugation as described under “Experimental Procedures.” One-twentieth of the total volume of each fraction was analyzed by SDS-PAGE and immunoblotted for PNPLA3, ADRP, and calnexin. A rabbit polyclonal antibody to the last 20 amino acids of human PNPLA3 was used to detect endogenous PNPLA3. B, postnuclear supernatants (PNS) prepared from HuH-7 cells stably expressing PNPLA3-V5 were subjected to ultracentrifugation at 100,000 × g to separate the cytoplasm (C) and membranes (M) as described under “Experimental Procedures.” One-twentieth of the total volume of each fraction was analyzed by SDS-PAGE, and immunoblotting using a V5 monoclonal antibody and rabbit anti-calnexin polyclonal antibody. C and D, membrane fractions from HuH-7 cells expressing wild type PNPLA3, PNPLA3-I148M (C), or truncated PNPLA3 (D) were suspended in 450 μl of 10 mm Tris, pH 7.4. Membranes were repelleted by centrifugation at 100,000 × g for 1 h at 4 °C and resuspended in the indicated buffers as described under “Experimental Procedures.” Pellet (P) and supernatant (S) fractions were subjected to 10% SDS-PAGE and analyzed by immunoblotting. E, lipid droplets were isolated from HuH-7 cells stably expressing recombinant PNPLA3 and PNPLA3-I148M. The postnuclear supernatant was adjusted to a sucrose concentration of 20%, applied to the bottom of a discontinuous sucrose gradient, and centrifuged at 28,000 × g as described under “Experimental Procedures.” A total of 20 μg of protein from the PNS and 17 μg from the lipid droplet fractions were subjected to immunoblotting using antibodies against V5 and ADRP, a lipid droplet marker. There experiments were repeated at least twice, and the results were similar.