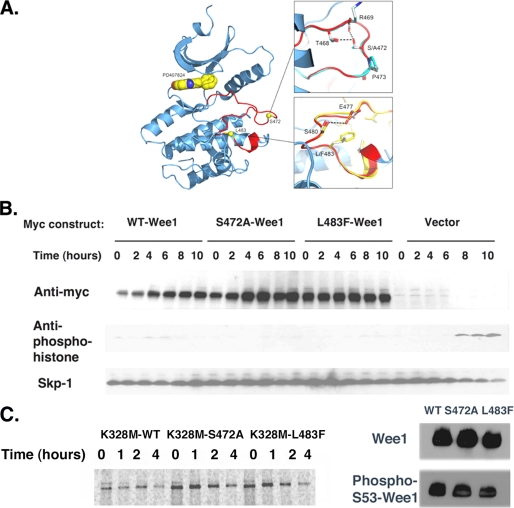
FIGURE 4.
S472A-Wee1 or L483F-Wee1 mutation does not affect Wee1 structure or kinase activity. A, X-ray crystal structure of the kinase domain of human Wee1 kinase highlighting the ATP-binding site and activation segment. The small molecule inhibitor PD407824 is drawn in the active site as a CPK model (space filling model by Corey et al. (29)). The insets show overlays of phenylalanine for leucine at position 483 and alanine for serine at position 472. The Wee1 activation domain is colored in red. B, HeLa cells were synchronized in the S phase using thymidine, released for 4 h, and transfected with Myc-WT-Wee1, Myc-S472A-Wee1, Myc-L483F-Wee1, or vector alone. Thymidine was then added for another 18 h, at which time the cells were released and isolated at 0, 2, 4, 6, 8, and 10 h and processed for Myc, anti-phosphohistone, and Skp1 immunoreactivity. C, left panel, pulse-chase analysis of Myc-K328M-Wee1, Myc-K328M-S472A-Wee1, or Myc-K328M-L483F-Wee1 in HeLa cells. Right panel, anti-Wee1 or phosphoserine 53-Wee1 immunoreactivity for wild-type (WT) Wee1, S472A-Wee1, or L483F-Wee1. A representative assay of three independent experiments is shown.