FIGURE 3.
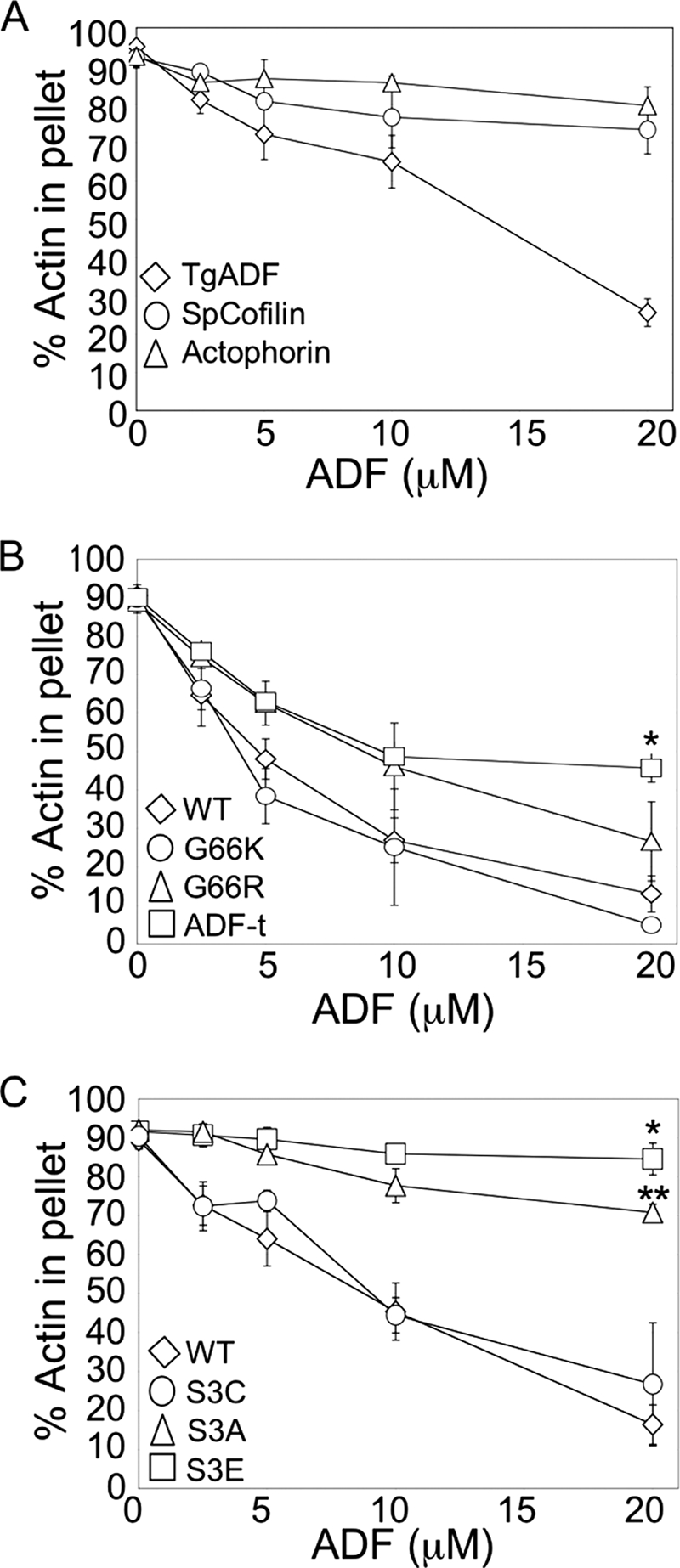
Comparison of TgADF activity with other ADF/Cofilin proteins, and mutational analysis of actin binding sites. A, comparison of TgADF activity with ADF/Cofilin proteins S. pombe cofilin and A. castellani actophorin. Quantitation of the proportion of actin sedimenting at 100,000 × g after polymerization by the addition of F buffer and incubation with TgADF, S. pombe cofilin (SpCofilin) or A. castellani actophorin (Actophorin). Experiments were done as described in Fig. 2. (n = 3 experiments, mean ± S.E.). B, effect of putative F-actin binding sites on TgADF activity. The filament disassembly activity of TgADF expressing the conserved basic F-loop residue (G66K or G66R) or the C-terminal residues of S. pombe cofilin (ADF-t) was compared with wild-type (WT) TgADF. The graph shows the relative proportion of actin sedimenting at 100,000 × g (n = 3 experiments, mean ± S.E.; *, p < 0.005 Student's t test, ADF-t versus WT). C, activity of TgADF serine 3 mutants. Actin filament disassembly activity of TgADF with mutations at the serine 3 residue to cysteine (S3C), alanine (S3A), or glutamic acid (S3E), were compared with WT TgADF (WT). The graph shows the relative proportion of actin sedimenting at 100,000 × g (n = 3 experiments, mean ± S.E.; *, p < 0.001 Student's t test, S3E versus WT; **, p < 0.05 Student's t test, S3A versus S3E).
