Abstract
Ecological theory traditionally predicts that interspecific competition selects for an increase in ecological specialization. Specialization, in turn, is often thought to be an evolutionary ‘dead end,’ with specialist lineages unlikely to evolve into generalist lineages. In host–parasite systems, this specialization can take the form of host specificity, with more specialized parasites using fewer hosts. We tested the hypothesis that specialists are evolutionarily more derived, and whether competition favours specialization, using the ectoparasitic feather lice of doves. Phylogenetic analyses revealed that complete host specificity is actually the ancestral condition, with generalists repeatedly evolving from specialist ancestors. These multiple origins of generalists are correlated with the presence of potentially competing species of the same genus. A competition experiment with captive doves and lice confirmed that congeneric species of lice do, in fact, have the potential to compete in ecological time. Taken together, these results suggest that interspecific competition can favour the evolution of host generalists, not specialists, over macroevolutionary time.
Keywords: specialization, coevolution, comparative methods, birds, lice
1. Introduction
Interspecific competition is generally believed to favour the evolution of ecological specialization. Studies of competition are particularly tractable in host–parasite systems where the host as a resource can be readily defined. In the case of parasites, ecological competition between species is thought to promote the evolution of specialization to particular host species, allowing parasites to avoid competition or become better competitors (Holmes 1973; Futuyma & Moreno 1988; Poulin 2007). Specialization is often considered to be an evolutionarily irreversible ‘dead end’ because host-specific parasites should have lower fitness on hosts to which they are not adapted (Cope 1896; Huxley 1942; Mayr 1963; Poulin 2007). In addition, the coevolutionary process itself may lead to ever-increasing host-specialization by parasites (Ehrlich & Raven 1964). Selection on parasites to avoid host defences should result in parasites becoming more specialized over time. However, several comparative studies have challenged the idea that host specialization is irreversible (Thompson 1994; Scheffer & Wiegmann 2000; Schluter 2000; Nosil 2002; Poulin et al. 2006; Simkova et al. 2006). We tested this hypothesis by reconstructing the evolution of host specialization in a large genus of parasitic lice (Phthiraptera), which are obligate vertebrate parasites well known for their host specificity.
Lice are ectoparasites of birds and mammals that complete their entire life cycle on the body of the host. They feed on feathers, skin or blood, and 67 per cent of all species are confined to a single host species (Durden & Musser 1994; Price et al. 2003). However, considerable variation exists in host specificity, with some species of lice parasitizing more than 50 species of hosts (Price et al. 2003). The feather lice parasitizing doves (Columbiformes), genus Columbicola, show considerable variation in host specificity (Bush et al. 2009), making them a good system for examining evolutionary changes in this trait. One factor that may influence host specificity is the competition between species of lice for food or space (Bush & Malenke 2008). The potential for competition between species of Columbicola exists because of the 157 dove species with a known species of Columbicola, 34 (22%) are host to at least one additional species of Columbicola (Bush et al. 2009). The goals of our study were to evaluate (i) the direction of changes in host specificity and (ii) whether these changes are related to the presence of potential competitors.
2. Material and methods
For comparative analyses of host specificity and competition, we used both a parsimony consensus tree and the best Bayesian tree resulting from phylogenetic analyses of two mitochondrial (COI and 12S rDNA) and one nuclear (elongation factor-1alpha) gene for Columbicola (trees from Johnson et al. 2007). All of the comparative analyses were conducted using both trees to assess the stability of our results to different methods of tree reconstruction. These trees included 46 of the 88 described species of Columbicola. In several cases, species that were previously believed to be host generalists were shown to be cryptic species that are actually host specific (Johnson et al. 2002, 2007; Malenke et al. 2009). In these cases, we used these cryptic host-specific species as terminal taxa and designated them with numbers according to previous publications (Johnson et al. 2007; Bush et al. 2009).
Species of Columbicola represented in our study occurred on one to four species of hosts, with the majority of species (84%) being completely specific to one host. To best represent this pattern, we coded each taxon as a host specialist if it occurred on only one host species and as a generalist if it occurred on more than one host species (Humphery-Smith 1989). This coding was based on the host–parasite associations for which we had molecular data (from Johnson et al. 2007). We reconstructed the evolution of host specificity as a discrete character using both parsimony (MacClade, Maddison & Maddison 1999) and maximum-likelihood (Mesquite, Maddison & Maddison 2006) techniques, with trees and branch lengths from both the parsimony consensus tree and the best Bayesian tree (Johnson et al. 2007). We evaluated the number and direction of character state changes in host specificity. To evaluate the phylogenetic conservation in host specificity, we randomly assigned character states to terminal taxa 1000 times and compared the actual number of changes with the number of changes observed in these randomizations (Maddison & Slatkin 1991). For likelihood reconstructions, we computed the marginal probability that the ancestral parasite was a generalist for each node (using the Mk1 model in Mesquite, Maddison & Maddison 2006).
To test whether the evolutionary changes in host specificity were associated with the presence of a potential competitor, we first scored whether each species of louse shared at least one of its hosts with another species of Columbicola (using host associations from Bush et al. 2009). We then used the concentrated changes test (Maddison 1990) to evaluate whether changes in host specificity were significantly associated with lineages that were inferred by parsimony to have a potentially competing species of Columbicola present. As a second analysis, we used the maximum-likelihood method of Pagel (1994) to assess whether there was a significant association between host specificity and the presence of a potentially competing species (using Mesquite). We conducted both of these analyses using both the parsimony and best Bayesian trees (Johnson et al. 2007) to assess the sensitivity of our results to tree topology.
Because generalist parasites, by definition, occur on more than one host, they may be more likely, purely by chance, to occur on a host that harbours a potentially competing parasite species. We controlled for this possible confounding factor by determining whether the association between host generalists and the presence of potential competitors was greater than that expected by chance, after taking their wider host distribution into account. We assigned each host species a 0 if it had one louse species or a 1 if it had more than one (Bush et al. 2009). We then randomized these binary codes across the host list 100 times. For each of these randomizations, we next scored whether each parasite occurred on a host with another parasite or not. The concentrated changes test (Maddison 1990) was then repeated for each randomization, using the parasite parsimony tree, addressing whether the changes to host generalist were concentrated in clades that had a potential competitor present. We compared the p-value from the concentrated changes test using the actual data to the distribution of p-values from the randomizations to assess the overall significance of our results after taking into account the fact that generalist parasites, by definition, are more widespread.
Another possible confounding factor is that, although we had very broad geographic and host sampling for lice, we were not able to sample fresh tissues of all known host associations for molecular phylogenetic analysis. Thus, we may have underestimated the number of generalist parasites, because some species of Columbicola are considered generalists on the basis of morphological taxonomy yet were only sampled from a single host in our study (and thus would have been coded as specialists in our analyses above). In these cases, we performed an alternative coding in which we ‘forced’ all of these species to be coded as generalists, and we re-ran the analyses using both trees and both the concentrated changes test (Maddison 1990) and maximum-likelihood correlation (Pagel 1994) techniques. Note that these species may actually represent host-specific cryptic species, as we have found for several other supposed ‘generalists’ (Johnson et al. 2007; Malenke et al. 2009) that were actually multiple, distinct host specialists (see above). However, this coding represents a conservative alternative for unsampled host associations.
To test whether species of Columbicola have the potential to compete, we performed an experiment in which we manipulated populations of Columbicola baculoides and Columbicola macrourae, both of which naturally parasitize mourning doves (Zenaida macroura). We used an additive experimental design with constant focal densities to keep intraspecific competitive effects the same (Goldberg & Scheiner 1993). We used 36 individually caged mourning doves as hosts in the experiment. Prior to the experiment, birds were housed in low humidity rooms (less than 30% relative humidity) for 10 weeks or more to clear any existing louse infestations (Harbison et al. 2008). After this period of low humidity, 12 birds were infested with 25 C. baculoides each, 12 were infested with 25 C. macrourae each and 12 were infested with 25 C. baculoides and 25 C. macrourae each. Twenty-five lice are approximately the number found on wild mourning doves (xmean = 23.8; n = 15).
The 12 replicates, each consisting of three individually caged birds, were haphazardly and evenly divided into two animal rooms (one bird escaped during the experiment, leaving 11 balanced replicates). The rooms were maintained on a 12 h light–dark schedule. The temperature was relatively constant at 18°C, and the mean daily relative humidity varied from 36 per cent to 75 per cent. The experiment ran for six weeks, which is about two louse generations (Bush & Clayton 2006). At the end of this period, all birds were euthanized and their louse populations quantified using a body washing method that accounts for 99 per cent of the lice on a bird (Clayton & Drown 2001).
3. Results and discussion
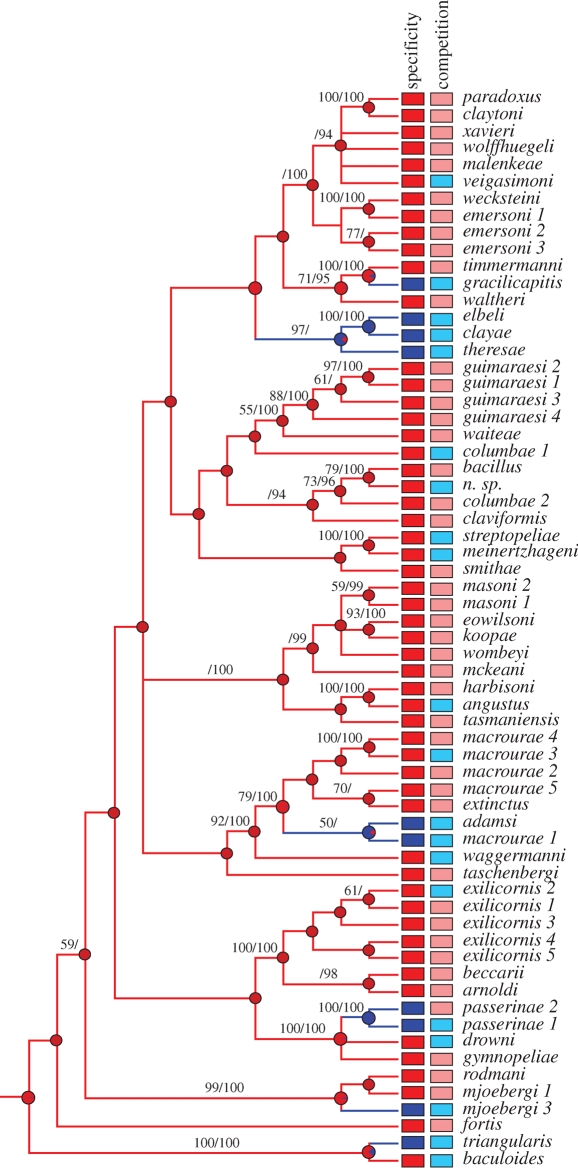
Evolutionary reconstruction of changes in host specificity in Columbicola suggests the hypothesis that, contrary to conventional wisdom, complete host specialization is the ancestral condition in this genus, and generalists have evolved from specialist ancestors multiple times. Parsimony reconstruction of host specificity over a molecular phylogeny (from Johnson et al. 2007) identified six cases of generalists evolving from specialists (figure 1). Surprisingly, there were no instances of specialists being derived from generalists. Similarly, a maximum-likelihood reconstruction of host specificity over the phylogenetic tree indicated the same six origins of generalists from specialists. Using maximum likelihood, the marginal probability of the ancestor of all dove wing lice being a specialist was 99 per cent (figure 1). Of the 52 nodes that were not a direct ancestor to two generalist descendants, 50 had a marginal probability greater than 95 per cent of being a specialist. Thus, it appears that the evolution of host generalization in these lice is nearly always, if not always, derived from host specialization.
Figure 1.
Comparative analysis of changes in host specificity (first column) and the presence of potential competitors (second column). Parsimony reconstruction of host specificity (first column: red, host specialist; blue, host generalist) over strict consensus parsimony tree (Johnson et al. 2007) for species of dove wing lice (Columbicola). Over this tree, there are six gains of host generalists from host specific ancestors. Pie charts on each branch are marginal probabilities, from maximum likelihood, of the ancestral state being specialist (red) or generalist (blue). These gains of host generalization were highly correlated with the presence of a potentially competing species of Columbicola (second column: light red, no other species present; light blue, another species present) on at least one of the host species (concentrated changes test (Maddison 1990), p < 0.003 for parsimony and Bayesian trees, maximum likelihood correlation (Pagel 1994), p < 0.01 over both trees). Numbers above branches are support from 1000 parsimony bootstrap replicates/Bayesian posterior probabilities. Only values greater than 50 per cent and 90 per cent, respectively, are shown.
We examined the relationship of these evolutionary changes in host specificity to the presence of potential competitors. Five of six changes from host specialist to host generalist occurred on branches that also had a potential competitor reconstructed (i.e. the lineage of Columbicola in question shares at least one of its hosts with another species of Columbicola). These changes were substantially more than that expected by chance using the concentrated changes test (p < 0.01, Maddison 1990). The association between host generalists and the presence of potentially competing species was also significant using maximum-likelihood character correlation methods (p < 0.01, Pagel 1994). We tested for potential statistical artifacts using additional randomization tests and alternate character codings. After randomizing host associations to control for the fact that generalist parasites, by definition, are more widespread, we found that our p-value (0.0011) from the original test was lower than all but two of the 100 randomizations. Thus, the correlation we observed was still more significant than would be expected by chance even after taking this into account. In addition, we controlled for unsampled host associations using a conservative coding of host generalists and found that all these tests were still statistically significant for both the concentrated changes test (p < 0.0001) and maximum-likelihood character correlation (p < 0.02) over both trees. Thus, even after taking into account potentially unsampled generalists, our results are still significant.
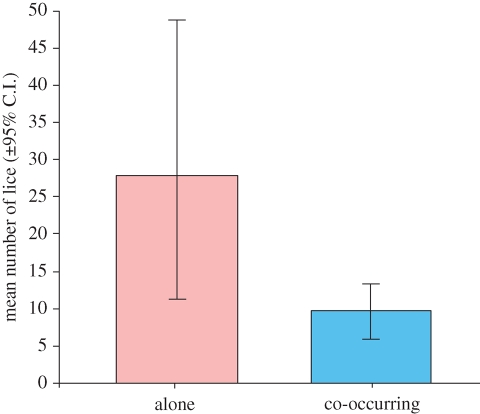
While it is impossible to experimentally manipulate existing macroevolutionary patterns, it is important to demonstrate the feasibility of putative underlying mechanisms using experiments. There have been few tests of interspecific competition in feather lice (Bush & Malenke 2008) and none, to our knowledge, of competition between congeneric species of lice. We therefore conducted an experiment with two species of Columbicola that occur on mourning doves (Z. macroura). Our experimental manipulations revealed that the population sizes of these species of lice are significantly lower when they co-occur than when they are alone on the host, and the magnitude of the interspecific competitive effect was similar for both species (figure 2). This reduction in population size is unlikely to be due to an increase in host defence (preening), which might be expected in response to experimental infestation with twice as many lice (25 of each species of louse, compared with 25 of a single species in the other two treatments). Control of Columbicola by preening is density independent (Clayton 1990); the reason for this appears to be that Columbicola cause no irritation, being confined to feathers rather than the host's skin (Clayton 1991). In summary, our experimental results confirm that different species of Columbicola can, in fact, compete. Our comparative tests examining macroevolutionary correlations, combined with this competition experiment, strongly suggest that competition favors generalization, counter to conventional wisdom.
Figure 2.
Competition between co-occurring species of lice. Populations of a given louse species were larger on hosts with a single louse species than on hosts with co-occurring species of lice (Wilcoxon signed-rank test; d.f. = 21, z = −2.33, p < 0.02). Each bar is the mean of 22 louse populations (11 C. baculoides and 11 C. macrourae). Analysis of each species independently indicated a similar magnitude of population reduction from interspecific competition (p = 0.04 and 0.068, giving a Fisher's combined probability of p = 0.02). Error bars are 95 per cent bootstrap confidence intervals (2000 replicates, Reiczigel & Rozsa 2005).
Although competition may select for generalists, other factors are likely to limit the evolution of host generalization. For example, Columbicola escape from host preening by hiding between the barbs of the host's feathers (Clayton et al. 2003). Lice are unable to escape from preening on hosts that differ too much in size from the native host because escape efficiency is influenced by feather size (Johnson et al. 2005; Bush & Clayton 2006). The availability of hosts of similar size in a particular geographical region should therefore limit the evolution of host generalists. Such factors may explain why, overall, host generalization shows significant phylogenetic conservation. The number of parsimony reconstructed changes over the Columbicola tree was fewer than expected by chance (p < 0.01, Maddison & Slatkin 1991), indicating that host specificity has phylogenetic signal and that host generalists tend to be concentrated in certain clades. These limitations may explain why some species of Columbicola share a host with a congener, yet have not become generalists (figure 1).
In cases where sharing a host with a congener does lead to change in the degree of specialization, our results indicate that the direction of change is from specialist to generalist, not the other way around. Thus, host specialization is not an evolutionary dead end, but instead lice can take advantage of opportunities to parasitize multiple host species. One way in which they can do this is by switching to new hosts, while continuing to parasitize the original host, a process known as ‘incomplete’ host switching (Johnson et al. 2003). Species of Columbicola are capable of switching between sympatric species of hosts by hitchhiking rides phoretically on parasitic flies (Hippoboscidae, Harbison et al. 2008, 2009). These flies are generally less specific than lice and provide a mechanism for louse dispersal among sympatric host species. Another way in which lice could add hosts is through ‘failure to speciate’ processes, in which gene flow is maintained between populations of parasites on speciating host populations (Johnson et al. 2003), again possibly involving phoretic hitchhiking.
Importantly, the evolution of host generalists in Columbicola is correlated with the presence of potentially competing species on the same host species. Rather than promoting an increase in host specialization, competition seems to favour the evolution of generalization, presumably as a way of reducing the intensity of competition. The proportion of host individuals in a population infested with Columbicola is seldom 100 per cent and is often less than 50 per cent (Moyer et al. 2002). Thus, it may well be possible for lice to escape from competition by dispersing to new host individuals that are free of lice. The availability of competitor free space should select for increased dispersal ability in species that suffer from interspecific competition. Such a competition–colonization trade-off can explain why generalist species are derived from specialists and why these origins are correlated with the presence of potentially competing species.
Acknowledgements
All procedures followed guidelines of the Institutional Animal Care and Use Committee of the University of Utah (05-08009).
We thank S. Bush, P. Coley, C. Dietrich, M. Douglas, G. Levin, A. Paterson, J. Weckstein and an anonymous reviewer for helpful comments on this manuscript. This study was supported by grants from NSF DEB-0107891 and DEB-0612938 to K.P.J., DEB-0608329 to J.R.M. and D.H.C. and DEB-0107947 and DEB-0614565 to D.H.C.
References
- Bush S. E., Clayton D. H.2006The role of body size in host specificity: reciprocal transfer experiments with feather lice. Evolution 60, 2158–2167 (doi:10.1554/06-226.1) [PubMed] [Google Scholar]
- Bush S. E., Malenke J. R.2008Host defence mediates interspecific competition in ectoparasites. J. Anim. Ecol. 77, 558–564 [DOI] [PubMed] [Google Scholar]
- Bush S. E., Price R. D., Clayton D. H.2009Description of eight new species of Columbicola feather lice (Phthiraptera: Philopteridae) with a comprehensive world checklist. J. Parasitol. 95, 286–294 (doi:10.1645/GE-1799.1) [DOI] [PubMed] [Google Scholar]
- Clayton D. H.1990Mate choice in experimentally parasitized rock doves: lousy males lose. Am. Zool. 30, 286–294 [Google Scholar]
- Clayton D. H.1991Coevolution of avian grooming and ectoparasite avoidance. In Bird–parasite interactions: ecology, evolution, and behaviour (eds Loye J. E., Zuk M.), pp. 258–289 Oxford, UK: Oxford University Press [Google Scholar]
- Clayton D. H., Drown D. M.2001Critical evaluation of five methods for quantifying chewing lice (Insecta: Phthiraptera). J. Parasitol. 87, 1291–1300 [DOI] [PubMed] [Google Scholar]
- Clayton D. H., Bush S. E., Goates B. M., Johnson K. P.2003Host defense reinforces host–parasite cospeciation. Proc. Natl Acad. Sci. USA 100, 15 694–15 699 (doi:10.1073/pnas.2533751100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope E. D.1896The primary factors of organic evolution. Chicago, IL: Open Court Publishing Co [Google Scholar]
- Durden L. A., Musser G. G.1994The sucking lice (Insecta, Anoplura) of the world: a taxonomic checklist with records of mammalian hosts and geographical distributions. Bull. Am. Mus. Nat. Hist. 218 [Google Scholar]
- Ehrlich P. R., Raven P. H.1964Butterflies and plants: a study of coevolution. Evolution 18, 586–608 (doi:10.2307/2406212) [Google Scholar]
- Futuyma D. A., Moreno G.1988The evolution of ecological specialization. Ann. Rev. Ecol. Syst. 19, 207–233 (doi:10.1146/annurev.es.19.110188.001231) [Google Scholar]
- Goldberg D. E., Scheiner S. M.1993ANOVA and ANCOVA: field competition experiments. In Design and analysis of ecological experiments (eds Scheiner S. M., Gurevitch J.), pp. 69–93. New York, NY: Chapman and Hall [Google Scholar]
- Harbison C. W., Bush S. E., Malenke J. R., Clayton D. H.2008Comparative transmission dynamics of competing parasite species. Ecology 89, 3186–3194 (doi:10.1890/07-1745.1) [DOI] [PubMed] [Google Scholar]
- Harbison C. W., Jacobsen M. V., Clayton D. H.2009Hitchhiker's guide to parasite transmission: phoretic behavior of feather lice. Int. J. Parasitol. 39, 569–575 (doi:10.1016/j.ijpara.2008.09.014) [DOI] [PubMed] [Google Scholar]
- Holmes J. C.1973Site segregation by parasitic helminthes: interspecific interactions, site segregation, and their importance to the development of helminth communities. Can. J. Zool. 51, 333–347 (doi:10.1139/z73-047) [DOI] [PubMed] [Google Scholar]
- Humphery-Smith I.1989The evolution of phylogenetic specificity among parasitic organisms. Parasitol. Today 5, 385–387 (doi:10.1016/0169-4758(89)90303-7) [DOI] [PubMed] [Google Scholar]
- Huxley J.1942Evolution: the modern synthesis London, UK: George Allen and Unwin [Google Scholar]
- Johnson K. P., Williams B. L., Drown D. M., Adams R. J., Clayton D. H.2002The population genetics of host specificity: genetic differentiation in dove lice (Insecta: Phthiraptera). Mol. Ecol. 11, 25–38 (doi:10.1046/j.0962-1083.2001.01412.x) [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Adams R. J., Page R. D. M., Clayton D. H.2003When do parasites fail to speciate in response to host speciation? Syst. Biol. 52, 37–47 (doi:10.1080/10635150390132704) [DOI] [PubMed] [Google Scholar]
- Johnson K. P., Bush S. E., Clayton D. H.2005Correlated evolution of host and parasite body size: tests of Harrison's Rule using birds and lice. Evolution 59, 1744–1753 [PubMed] [Google Scholar]
- Johnson K. P., Reed D. L., Hammond Parker S. L., Kim D., Clayton D. H.2007Phylogenetic analysis of nuclear and mitochondrial genes supports species groups for Columbicola (Insecta: Phthiraptera). Mol. Phylogenet. Evol. 45, 506–518 (doi:10.1016/j.ympev.2007.07.005) [DOI] [PubMed] [Google Scholar]
- Maddison W. P.1990A method for testing the correlated evolution of two binary characters: are gains or losses concentrated on certain branches of a phylogenetic tree? Evolution 44, 539–557 (doi:10.2307/2409434) [DOI] [PubMed] [Google Scholar]
- Maddison W. P., Maddison D. R.1999MacClade: analysis of phylogeny and character evolution, v. 3.08 Sunderland, MA: Sinauer Associates [Google Scholar]
- Maddison W. P., Maddison D. R.2006Mesquite: a modular system for evolutionary analysis, v. 1.11 See http://mesquiteproject.org [Google Scholar]
- Maddison W. P., Slatkin M.1991Null models for the number of evolutionary steps in a character on a phylogenetic tree. Evolution 45, 1184–1197 (doi:10.2307/2409726) [DOI] [PubMed] [Google Scholar]
- Malenke J. R., Johnson K. P., Clayton D. H.2009Host specialization differentiates cryptic species of feather-feeding lice. Evolution 63, 1427–1438 (doi:10.1111/j.1558-5646.2009.00642.x) [DOI] [PubMed] [Google Scholar]
- Mayr E.1963Animal species and evolution Cambridge, MA: Harvard University Press [Google Scholar]
- Moyer B. R., Drown D. M., Clayton D. H.2002Low humidity reduces ectoparasite pressure: implications for host life history evolution. Oikos 97, 223–228 (doi:10.1034/j.1600-0706.2002.970208.x) [Google Scholar]
- Nosil P.2002Transition rates between specialization and generalization in phytophagous insects. Evolution 56, 1701–1706 [DOI] [PubMed] [Google Scholar]
- Pagel M.1994Detecting correlated evolution on phylogenies: a general method for the comparative analysis of discrete characters. Proc. R. Soc. Lond. B 255, 37–45 (doi:10.1098/rspb.1994.0006) [Google Scholar]
- Poulin R.2007Evolutionary ecology of parasites, 2nd edn Princeton, NJ: Princeton University Press [Google Scholar]
- Poulin R., Krasnov B. R., Shenbrot G. I., Mouillot D., Khoklhova I. S.2006Evolution of host specificity in fleas: is it directional and irreversible? Int. J. Parasitol. 36, 185–191 (doi:10.1016/j.ijpara.2005.09.017) [DOI] [PubMed] [Google Scholar]
- Price R. D., Hellenthal R. A., Palma R. L., Johnson K. P., Clayton D. H.2003The chewing lice: world checklist and biological overview. Ill. Nat. Hist. Surv. Spec. Publ. 24 [Google Scholar]
- Reiczigel J., Rozsa L.2005Quantitative parasitology 3.0 Budapest, Hungary: distributed by the authors [Google Scholar]
- Scheffer S. J., Wiegmann B. M.2000Molecular phylogenetics of the holly leafminers (Diptera: Agromyzidae: Phytomyza): species limits, speciation, and dietary specialization. Mol. Phylogenet. Evol. 17, 244–255 (doi:10.1006/mpev.2000.0830) [DOI] [PubMed] [Google Scholar]
- Schluter D.2000The ecology of adaptive radiation Oxford, UK: Oxford University Press [Google Scholar]
- Simkova A., Verneau O., Gelnar M., Morand S.2006Specificity and specialization of congeneric monogeneans parasitizing cyprinid fish. Evolution 60, 1023–1037 [PubMed] [Google Scholar]
- Thompson J. N.1994The coevolutionary process Chicago, IL: University of Chicago Press [Google Scholar]