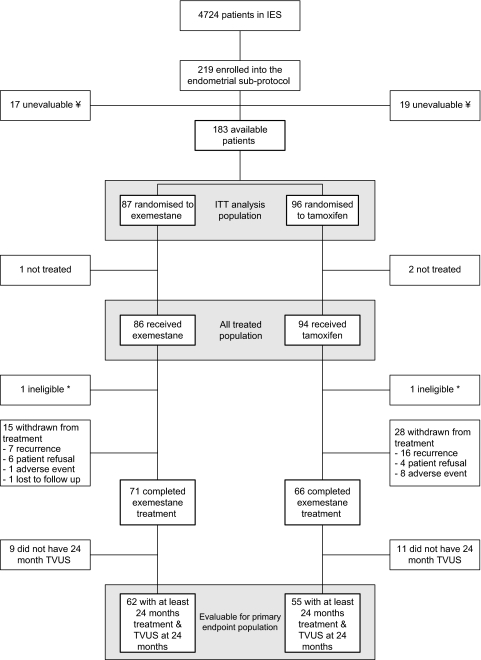
Figure 1.
Consolidated standards of reporting trials diagram. ¥Monitoring carried out by the sponsor highlighted a number of issues regarding the conduct of the endometrial sub-protocol at a satellite site. Based on these findings, patients at this satellite site have been excluded from the endometrial sub-protocol analysis (n = 36). *TVUS not carried out ≤30 days before randomisation. These patients were included in the analyses presented here. TVUS, transvaginal ultrasound; IES, Intergroup Exemestane Study; ITT, intention-to-treat.