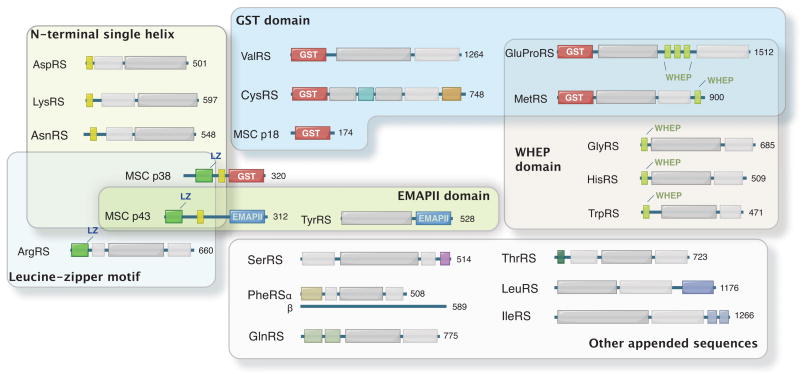
Figure 1.
Appended domain/motifs in human AARSs and MSC-associated factors. Except for human AlaRS, all AARSs have appended domains compared to their bacterial or archaeal homologues. The prokaryotic catalytic domains and tRNA recognition domains are shown as white and gray boxes. N-terminal single helices are present in three class IIb AARSs (yellow boxes). Homologous GST domains are shown in the N-terminal regions of MetRS, GluProRS, ValRS and CysRS, as well as in the C-terminal regions of MSC p18 and p38. MSC p38 also contains a leucine zipper motif (LZ) (green box) and is involved in interactions with leucine zippers of MSC p43 and ArgRS.(Min—in the figure, replace p38/AIMP2 with MSC p38, and same for p43.) The EMAPII domains (blue boxes) in MSC p43 and TyrRS are related. Homologous WHEP domains are shared by five human AARSs. Many other appended domains in AARSs are idiosyncratic to each enzyme and remain to be further characterized.