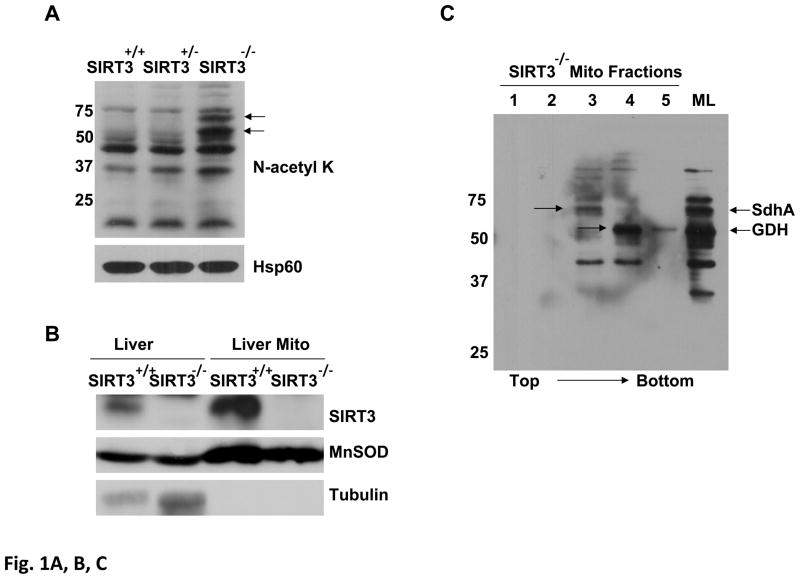
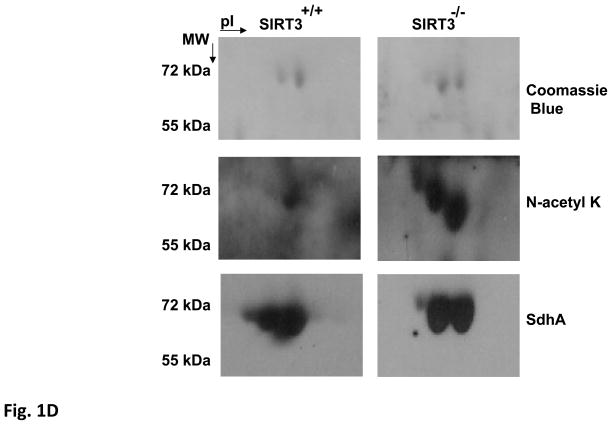
Figure 1. Detection of SdhA as a novel SIRT3 substrate in SIRT3 knock-out mice liver mitochondria.
Acetylated proteins and SIRT3 expression in Sirt3+/+, Sirt3+/−, and Sirt3−/− mice liver mitochondria were evaluated by immunoblotting analysis using various antibodies. A) Mitochondrial lysates prepared as described in the Materials and Methods were separated on SDS-PAGE and increased acetylation of mitochondrial proteins was detected by immunoblotting probed with N-acetyl lysine (N-acetyl K) antibody. As a control for equal loading, protein blot was developed with Hsp60 antibody. B) SIRT3 protein levels in the liver or isolated liver mitochondria from wild type or SIRT3 deficient mice were detected by immunoblotting analysis using SIRT3 antibody. Mitochondrial protein MnSOD and cytoplasmic protein tubulin were also detected as controls. C) Approximately, 2mg of Sirt3−/− mice liver mitochondrial lysate were layered on 34% sucrose cushion and fractioned into five separate layers (the top and bottom fractions of the sucrose cushion is shown by the arrow). Equal volumes of each fraction were separated on SDS-PAGE gel and acetylated proteins in each fraction were detected by immunoblotting analysis. The total Sirt3−/− mice liver mitochondrial lysate (ML) layered on the cushion was also analyzed to locate the acetylated proteins in the fractions. Arrows show the location of SIRT3 substrates glutamate dehydrogenase (GDH) and the flavoprotein subunit of succinate dehydrogenase (SdhA). D) Approximately, 50 μl of fraction 3 from Sirt3+/+ or Sirt3−/− mice liver mitochonria was separated on 2D-gels and acetylated proteins were detected with N-acetyl lysine antibody. The acetylated 2D-gel spots corresponding to the Coomassie Blue stained gel spots were in-gel digested and identified by mass spectrometry. The protein identification determined by mass spectrometry was confirmed by immunoblotting using SdhA antibody.