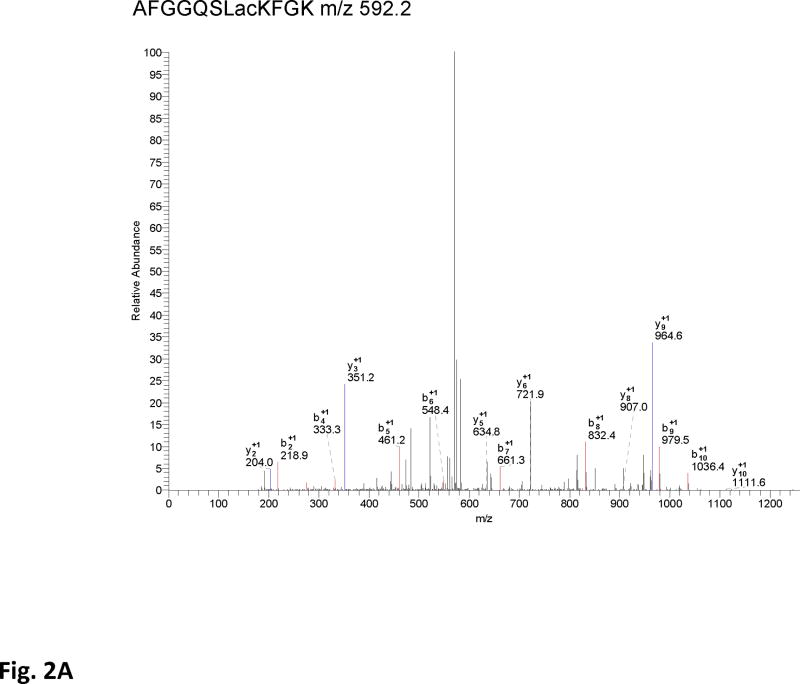
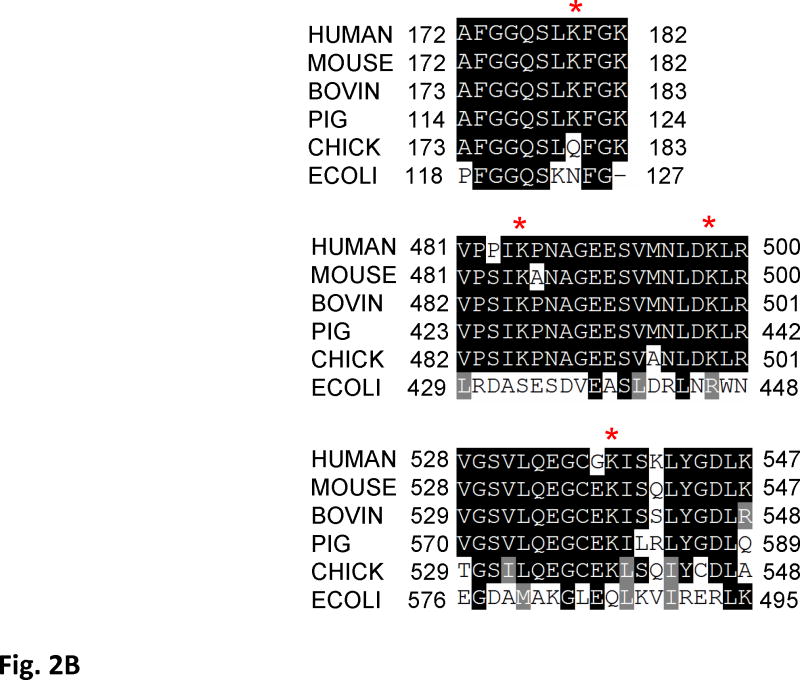
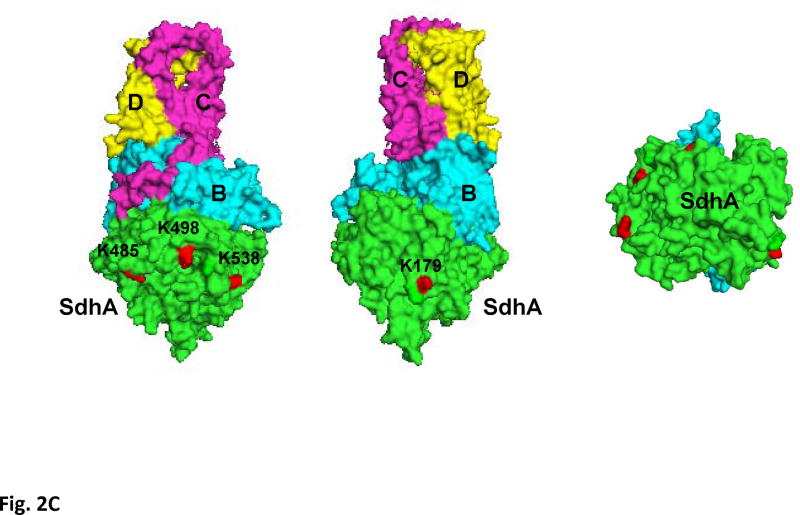
Figure 2. Acetylation of SdhA at conserved K179, K485, K498, and K538 residues.
A) The CID spectrum of acetylated peptide detected in LC-MS/MS analysis of 2D-gel spot of SdhA from SIRT3 knock-out mice mitochondria. B) Primary sequence alignment of acetylated peptides from mice SdhA and its homologs from different species. The human, bovine, pig, chicken, and E. coli SdhA were aligned with acetylated peptides of mouse SdhA. (*) denotes the acetylated Lys residues detected in the LC-MS/MS analysis. The alignment was created with CLUSTALW program in Biology Workbench and displayed in BOXSHADE. C) Crystal structure model of the chicken SdhA (PDB# 1YQ3) representing the all four subunits SdhA (green), SdhB (cyan), SdhC, and SdhD (yellow and pink, respectively). The conserved Lys residues found to be acetylated in mouse SdhA (shown by asterisks in B) were colored in red.