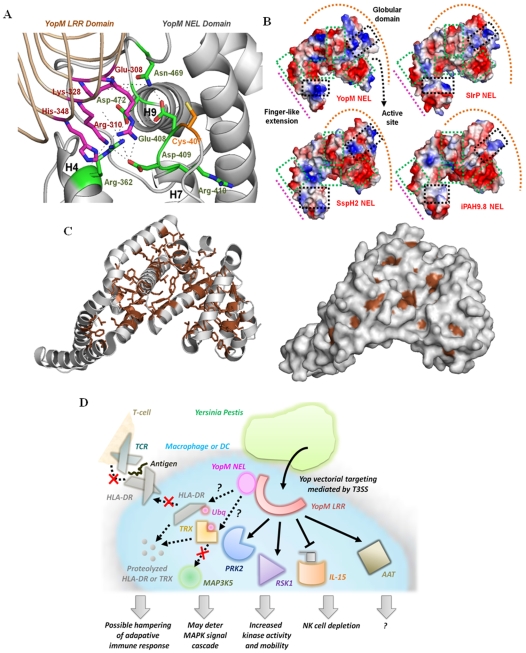
Figure 6. Application of the PCAIN methodology to analyze potential structure-function relationships of the novel E3 ligase (NEL) domain from the YopM effector protein of the plague-causative bacterium Yersinia Pestis.
(A.) The YopM NEL domain structure was modeled using the PCAIN methodology and the putative ubiquitin ligase catalytic site was characterized, based on the recent experimental characterizations of Salmonella and Shigella NEL domains [38]–[41]. The likely hydrogen bonds that stabilize the active site (black lines) and the key α-helices (H4, H7, and H9) are indicated. (B.) Vacuum electrostatics of the molecular surfaces from superposed NEL domains of YopM, SlrP, SspH2, and ipaH were generated (see Methods ) with negative, positive, and neutral patches colored red, blue, and white respectively. The finger-like extension (pink line), globular domain (orange arc), and active site location (black arrow) are indicated. (C.) The solvent-unexposed residues that constitute the PCAIN of the modeled YopM NEL domain structure (gray) are shown as sticks (brown). The molecular surface of the YopM NEL domain is also shown alongside to highlight that the residues constituting the PCAIN (brown) are only very minimally solvent exposed. (D.) This is a pictorial depiction of YopM in the intracellular context and the key structural implications for its modulation of human adaptive and innate immune signaling. Specifically, YopM is known to interact with protein kinase C-like 2 (PRK2) and ribosomal S6 protein kinase 1 (RSK1) resulting in increased activity and mobility of these kinases, in addition to potentiating natural killer (NK) cell depletion by suppressing expression of Interleukin-15 (IL-15) [37]. YopM has also been shown to specifically interact with α1-antitrypsin (AAT) without affecting its anti-protease activity, due to which the biological significance of this interaction remains unknown.[37] Also indicated by the question mark (?) symbols are hitherto unknown interactions for YopM, extrapolated based on the functions of the related proteins. Specifically highlighted in this regard are the degradation of human leukocyte antigen-DR (HLA-DR) and thioredoxin (TRX) that may cause suppression of adaptive immune response via moderation of antigen presentation and modulation of innate immune signaling via the MAPK cascade, respectively. It remains to be seen what precise intracellular molecules are targeted by YopM NEL for proteolytic degradation, considering the autoregulated ubiquitin ligase activity suggested by our PCAIN-based model and analysis.