Abstract
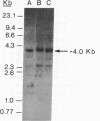
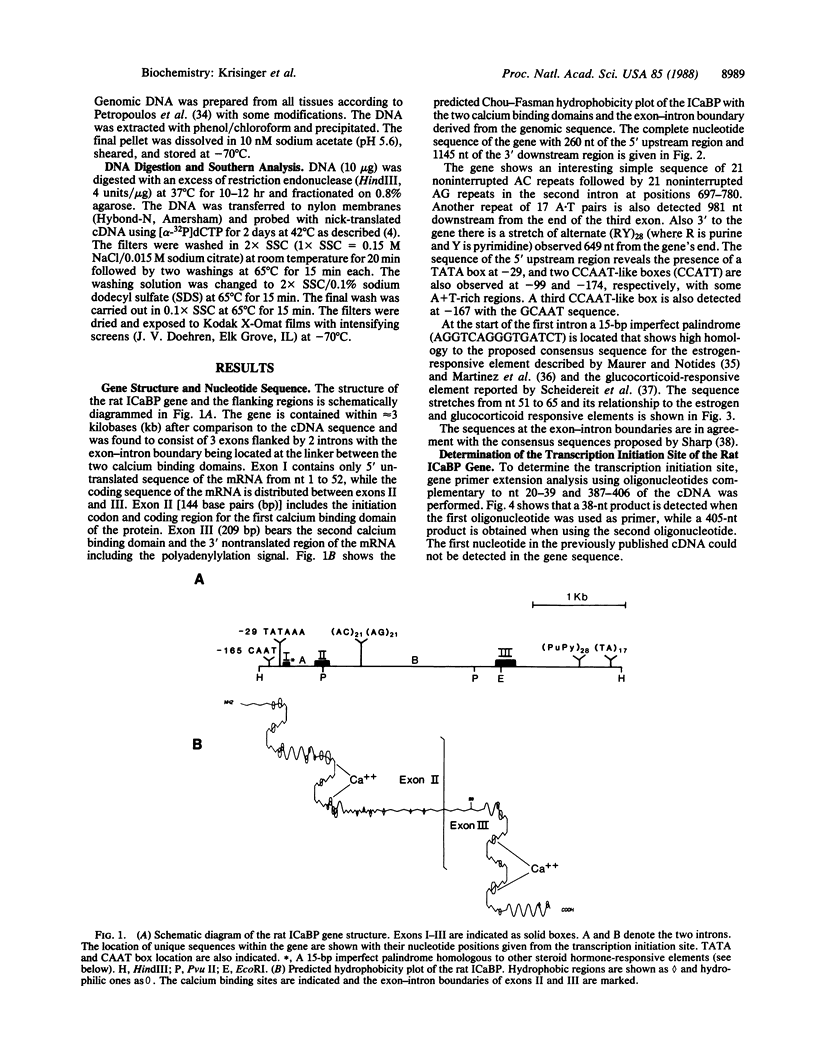
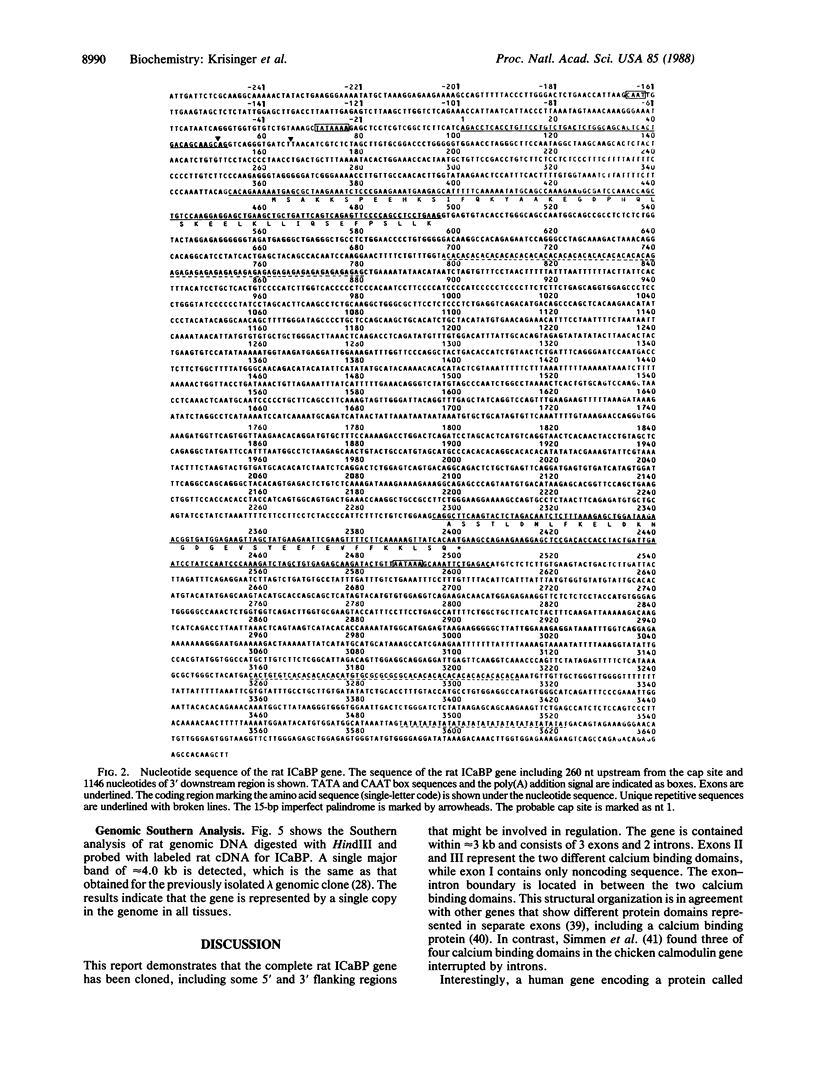
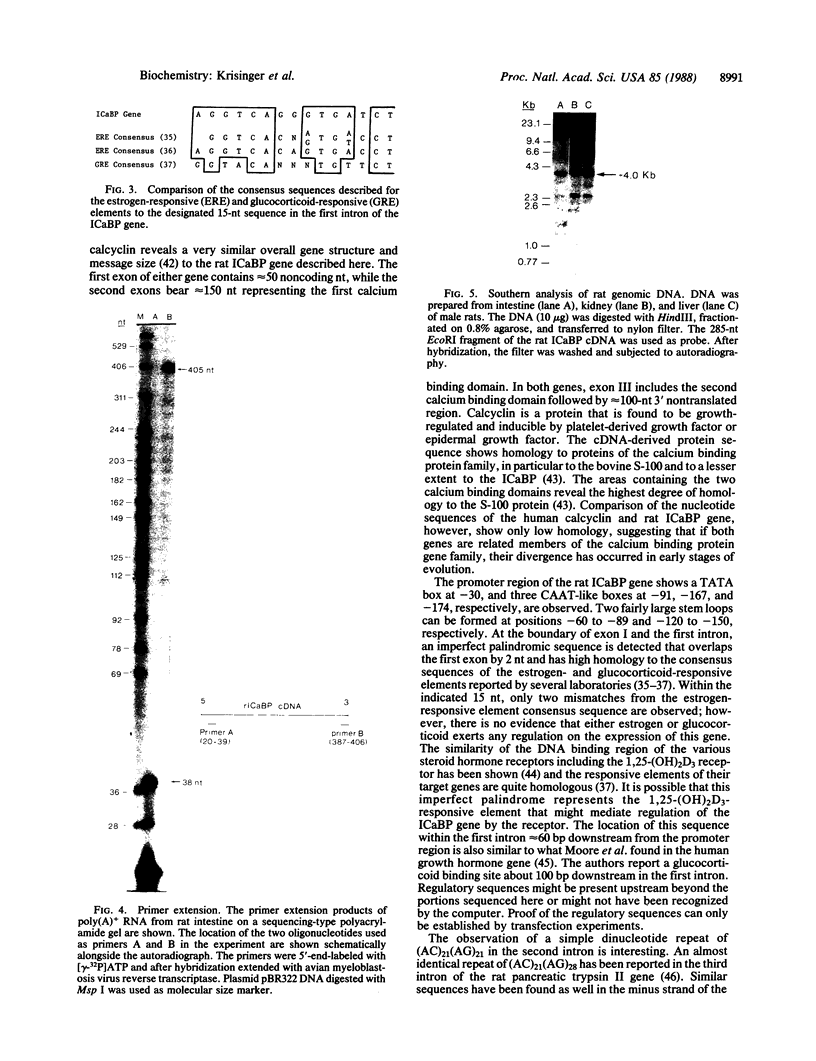
The vitamin D-dependent intestinal calcium binding protein (ICaBP, 9 kDa) is under transcriptional regulation by 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3], the hormonal active form of the vitamin. To study the mechanism of gene regulation by 1,25-(OH)2D3, we isolated the rat ICaBP gene by using a cDNA probe. Its nucleotide sequence revealed 3 exons separated by 2 introns within approximately 3 kilobases. The first exon represents only noncoding sequences, while the second and third encode the two calcium binding domains of the protein. The gene contains a 15-base-pair imperfect palindrome in the first intron that shows high homology to the estrogen-responsive element. This sequence may represent the vitamin D-responsive element involved in the regulation of the ICaBP gene. The second intron shows an 84-base-pair-long simple nucleotide repeat that implicates Z-DNA formation. Genomic Southern analysis shows that the rat gene is represented as a single copy.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudier J., Glasser N., Strid L., Brehier A., Thomasset M., Gerard D. Purification, calcium-binding properties, and conformational studies on a 28-kDa cholecalcin-like protein from bovine brain. J Biol Chem. 1985 Sep 5;260(19):10662–10670. [PubMed] [Google Scholar]
- Bredderman P. J., Wasserman R. H. Chemical composition, affinity for calcium, and some related properties of the vitamin D dependent calcium-binding protein. Biochemistry. 1974 Apr 9;13(8):1687–1694. doi: 10.1021/bi00705a021. [DOI] [PubMed] [Google Scholar]
- Bruns M. E., Fausto A., Avioli L. V. Placental calcium binding protein in rats. Apparent identity with vitamin D-dependent calcium binding protein from rat intestine. J Biol Chem. 1978 May 10;253(9):3186–3190. [PubMed] [Google Scholar]
- Calabretta B., Battini R., Kaczmarek L., de Riel J. K., Baserga R. Molecular cloning of the cDNA for a growth factor-inducible gene with strong homology to S-100, a calcium-binding protein. J Biol Chem. 1986 Sep 25;261(27):12628–12632. [PubMed] [Google Scholar]
- Christakos S., Bruns M. E., Mehra A. S., Rhoten W. B., Van Eldik L. J. Calmodulin and rat vitamin D-dependent calcium-binding proteins: biochemical and immunochemical comparison. Arch Biochem Biophys. 1984 May 15;231(1):38–47. doi: 10.1016/0003-9861(84)90360-6. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Choo Q. L., Swift G. H., Quinto C., MacDonald R. J., Rutter W. J. Structure of two related rat pancreatic trypsin genes. J Biol Chem. 1984 Nov 25;259(22):14255–14264. [PubMed] [Google Scholar]
- Darwish H. M., Krisinger J., Strom M., DeLuca H. F. Molecular cloning of the cDNA and chromosomal gene for vitamin D-dependent calcium-binding protein of rat intestine. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6108–6111. doi: 10.1073/pnas.84.17.6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie M. Calcium-ion-binding activity in human small-intestinal mucosal cytosol. Purification of two proteins and interrelationship of calcium-binding fractions. Biochem J. 1981 Jul 1;197(1):55–65. doi: 10.1042/bj1970055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A. C., Danan J. L., Acker M. G., Ripoche M. A., Mathieu H. In rat uterus 17 beta-estradiol stimulates a calcium-binding protein similar to the duodenal vitamin D-dependent calcium-binding protein. Endocrinology. 1983 Oct;113(4):1340–1347. doi: 10.1210/endo-113-4-1340. [DOI] [PubMed] [Google Scholar]
- Desplan C., Heidmann O., Lillie J. W., Auffray C., Thomasset M. Sequence of rat intestinal vitamin D-dependent calcium-binding protein derived from a cDNA clone. Evolutionary implications. J Biol Chem. 1983 Nov 25;258(22):13502–13505. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emori Y., Ohno S., Tobita M., Suzuki K. Gene structure of calcium-dependent protease retains the ancestral organization of the calcium-binding protein gene. FEBS Lett. 1986 Jan 6;194(2):249–252. doi: 10.1016/0014-5793(86)80094-1. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Calabretta B., deRiel J. K., Battini R., Ghezzo F., Lauret E., Griffin C., Emanuel B. S., Gurrieri F., Baserga R. Structural and functional analysis of a growth-regulated gene, the human calcyclin. J Biol Chem. 1987 Jun 15;262(17):8325–8332. [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. The amino acid sequence of bovine intestinal calcium-binding protein. J Biol Chem. 1981 Jun 10;256(11):5669–5674. [PubMed] [Google Scholar]
- Gilbert W. Genes-in-pieces revisited. Science. 1985 May 17;228(4701):823–824. doi: 10.1126/science.4001923. [DOI] [PubMed] [Google Scholar]
- Gross M. D., Nelsestuen G. L., Kumar R. Observations on the binding of lanthanides and calcium to vitamin D-dependent chick intestinal calcium-binding protein. Implications regarding calcium-binding protein function. J Biol Chem. 1987 May 15;262(14):6539–6545. [PubMed] [Google Scholar]
- Hofmann T., Kawakami M., Hitchman A. J., Harrison J. E., Dorrington K. J. The amino acid sequence of porcine intestinal calcium-binding protein. Can J Biochem. 1979 Jun;57(6):737–748. doi: 10.1139/o79-092. [DOI] [PubMed] [Google Scholar]
- Hunziker W. The 28-kDa vitamin D-dependent calcium-binding protein has a six-domain structure. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7578–7582. doi: 10.1073/pnas.83.20.7578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa M., Hosoi J., Hashiba H., Nose K., Tohyama C., Abe E., Suda T., Kuroki T. Regulation of metallothionein gene expression by 1 alpha,25-dihydroxyvitamin D3 in cultured cells and in mice. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8810–8813. doi: 10.1073/pnas.84.24.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M. A., Lamm L., Jarnagin K., DeLuca H. F. 1,25-Dihydroxyvitamin D3-stimulated mRNAs in rat small intestine. Arch Biochem Biophys. 1986 Dec;251(2):403–412. doi: 10.1016/0003-9861(86)90346-2. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Strauss A. W., Go M. F., Alpers D. H., Gordon J. I. Biosynthesis and compartmentalization of rat-intestinal vitamin-D-dependent calcium-binding protein. Eur J Biochem. 1984 Mar 15;139(3):561–571. doi: 10.1111/j.1432-1033.1984.tb08042.x. [DOI] [PubMed] [Google Scholar]
- MacManus J. P., Watson D. C., Yaguchi M. The purification and complete amino acid sequence of the 9000-Mr Ca2+-binding protein from rat placenta. Identity with the vitamin D-dependent intestinal Ca2+-binding protein. Biochem J. 1986 Apr 15;235(2):585–595. doi: 10.1042/bj2350585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Givel F., Wahli W. The estrogen-responsive element as an inducible enhancer: DNA sequence requirements and conversion to a glucocorticoid-responsive element. EMBO J. 1987 Dec 1;6(12):3719–3727. doi: 10.1002/j.1460-2075.1987.tb02706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Notides A. C. Identification of an estrogen-responsive element from the 5'-flanking region of the rat prolactin gene. Mol Cell Biol. 1987 Dec;7(12):4247–4254. doi: 10.1128/mcb.7.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A. Selective binding of the estradiol receptor to a region at least one kilobase upstream from the rat prolactin gene. DNA. 1985 Feb;4(1):1–9. doi: 10.1089/dna.1985.4.1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell D. P., Mangelsdorf D. J., Pike J. W., Haussler M. R., O'Malley B. W. Molecular cloning of complementary DNA encoding the avian receptor for vitamin D. Science. 1987 Mar 6;235(4793):1214–1217. doi: 10.1126/science.3029866. [DOI] [PubMed] [Google Scholar]
- Moore D. D., Marks A. R., Buckley D. I., Kapler G., Payvar F., Goodman H. M. The first intron of the human growth hormone gene contains a binding site for glucocorticoid receptor. Proc Natl Acad Sci U S A. 1985 Feb;82(3):699–702. doi: 10.1073/pnas.82.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansini A. R., Christakos S. Cell-free translational analysis of messenger ribonucleic acid coding for vitamin D-dependent rat renal calcium-binding protein. Endocrinology. 1985 Oct;117(4):1652–1660. doi: 10.1210/endo-117-4-1652. [DOI] [PubMed] [Google Scholar]
- Perret C., Lomri N., Gouhier N., Auffray C., Thomasset M. The rat vitamin-D-dependent calcium-binding protein (9-kDa CaBP) gene. Complete nucleotide sequence and structural organization. Eur J Biochem. 1988 Feb 15;172(1):43–51. doi: 10.1111/j.1432-1033.1988.tb13853.x. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Yaswen P., Panzica M., Fausto N. Methylation of the alphafetoprotein gene in cell populations isolated from rat livers during carcinogenesis. Nucleic Acids Res. 1985 Nov 25;13(22):8105–8118. doi: 10.1093/nar/13.22.8105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince C. W., Butler W. T. 1,25-Dihydroxyvitamin D3 regulates the biosynthesis of osteopontin, a bone-derived cell attachment protein, in clonal osteoblast-like osteosarcoma cells. Coll Relat Res. 1987 Sep;7(4):305–313. doi: 10.1016/s0174-173x(87)80036-5. [DOI] [PubMed] [Google Scholar]
- Rowe D. W., Kream B. E. Regulation of collagen synthesis in fetal rat calvaria by 1,25-dihydroxyvitamin D3. J Biol Chem. 1982 Jul 25;257(14):8009–8015. [PubMed] [Google Scholar]
- Scheidereit C., Westphal H. M., Carlson C., Bosshard H., Beato M. Molecular model of the interaction between the glucocorticoid receptor and the regulatory elements of inducible genes. DNA. 1986 Oct;5(5):383–391. doi: 10.1089/dna.1986.5.383. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Silver J., Russell J., Sherwood L. M. Regulation by vitamin D metabolites of messenger ribonucleic acid for preproparathyroid hormone in isolated bovine parathyroid cells. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4270–4273. doi: 10.1073/pnas.82.12.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen R. C., Tanaka T., Ts'ui K. F., Putkey J. A., Scott M. J., Lai E. C., Means A. R. The structural organization of the chicken calmodulin gene. J Biol Chem. 1985 Jan 25;260(2):907–912. [PubMed] [Google Scholar]
- Simpson R. U., Hsu T., Begley D. A., Mitchell B. S., Alizadeh B. N. Transcriptional regulation of the c-myc protooncogene by 1,25-dihydroxyvitamin D3 in HL-60 promyelocytic leukemia cells. J Biol Chem. 1987 Mar 25;262(9):4104–4108. [PubMed] [Google Scholar]
- Sogawa K., Gotoh O., Kawajiri K., Fujii-Kuriyama Y. Distinct organization of methylcholanthrene- and phenobarbital-inducible cytochrome P-450 genes in the rat. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5066–5070. doi: 10.1073/pnas.81.16.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970 Sep;100(9):1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- Szebenyi D. M., Obendorf S. K., Moffat K. Structure of vitamin D-dependent calcium-binding protein from bovine intestine. Nature. 1981 Nov 26;294(5839):327–332. doi: 10.1038/294327a0. [DOI] [PubMed] [Google Scholar]
- Takagi T., Nojiri M., Konishi K., Maruyama K., Nonomura Y. Amino acid sequence of vitamin D-dependent calcium-binding protein from bovine cerebellum. FEBS Lett. 1986 May 26;201(1):41–45. doi: 10.1016/0014-5793(86)80567-1. [DOI] [PubMed] [Google Scholar]
- Thomasset M., Cuisinier-Gleizes P., Mathieu H., DeLuca H. F. Intestinal calcium-binding protein (CaBP) and bone calcium mobilization in response to 1,24(R),25-(OH)3D3. Comparative effects of 1,25-(OH)2D3 and 24(R),25-(OH)2D3 in rats. Mol Pharmacol. 1980 May;17(3):362–366. [PubMed] [Google Scholar]
- Thomasset M., Parkes C. O., Cuisinier-Gleizes P. Rat calcium-binding proteins: distribution, development, and vitamin D dependence. Am J Physiol. 1982 Dec;243(6):E483–E488. doi: 10.1152/ajpendo.1982.243.6.E483. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wasserman R. H., Fullmer C. S. Calcium transport proteins, calcium absorption, and vitamin D. Annu Rev Physiol. 1983;45:375–390. doi: 10.1146/annurev.ph.45.030183.002111. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]