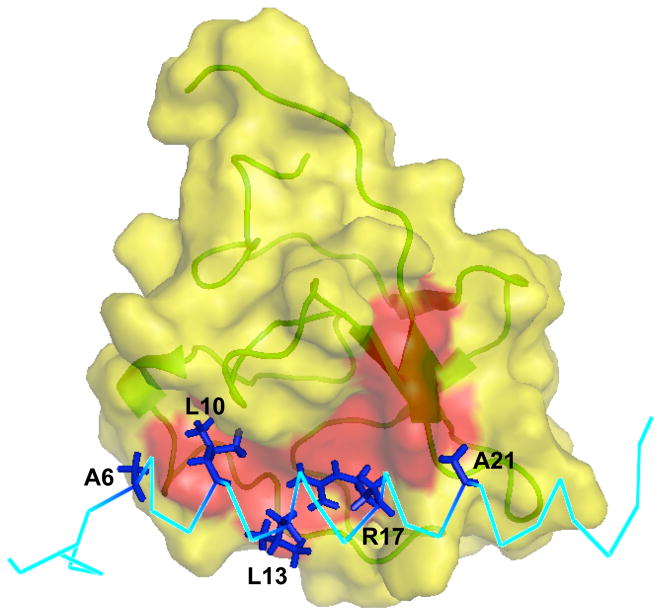
Fig. 3.
Surface representation of the binding interface between VEK-30 and K2Pg. The exposed hydrophobic groove (formed by the aromatic residues Tyr35, Phe40, Trp60, Phe62, Trp70 and Tyr72) on the surface of the K2Pg domain is highlighted red. The hydrophobic residues and Arg17 of VEK-30 involved are labeled and depicted as sticks (dark blue).