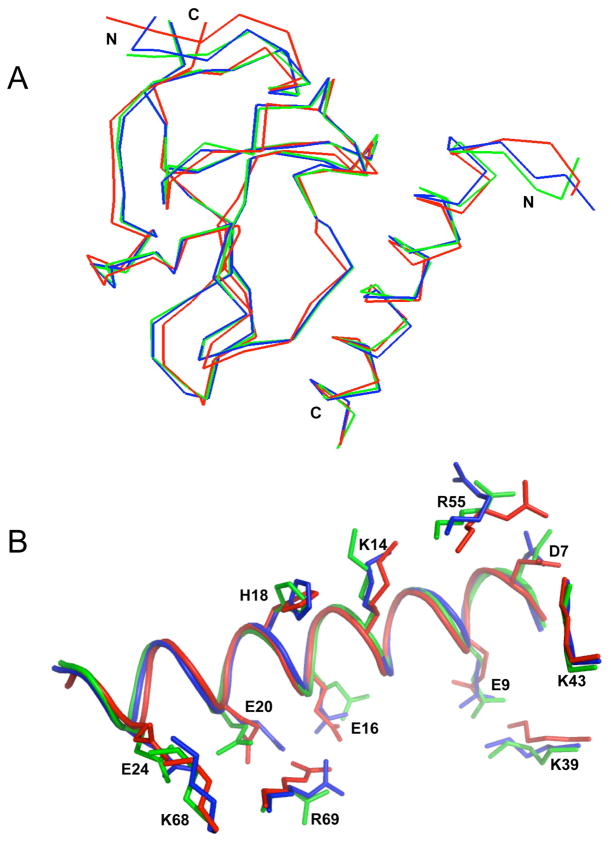
Fig. 5.
Superimposition of X-ray crystallographic and NMR solution structures of the VEK-30/K2Pg. (A). The backbone trace of two molecules (molecule-1 and molecule-2) in the unit cell of the crystal structure (PDB entry 115K), and the lowest energy NMR structure of VEK-30/K2Pg are overlaid and represented as blue, green and red lines, respectively. (B). Overlay of side-chain residues involved in the binding interactions. The intermolecular side-chain interactions of K2Pg and VEK-30 of molecule-1 (blue) and molecule-2 (green) of the X-ray crystal structure and the lowest energy NMR structure (red) are compared with side-chain orientations (represented as sticks). The main-chain of VEK-30 in the three structures is depicted as a ribbon diagram.