Abstract
The immature immune system requires constant stimulation by foreign antigens during the early stages of life to develop properly and to create efficient immune responses against later infections. We have previously shown that intake of antigenic dietary protein is critical for inducing maturation of the immune system as well as for the development of T helper type 1 (Th1) immunity. In this study, we show that administration of an amino acid (aa)-based diet during the development of the immune system subsequently resulted in inefficient control of Leishmania major infection in adult C57BL/6 mice. Compared with mice fed a control protein-containing diet, adult aa-fed mice showed a decreased interferon (IFN)-γ response to parasite antigens and insufficient production of nitric oxide (NO), which is crucial to parasite death. However, no deviation towards Th2-specific immunity to L. major was observed. Phenotypic analysis of antigen-presenting cells (APCs) from aa-fed mice revealed deficient levels of the costimulatory molecules CD40 and CD80, and low levels of interleukin (IL)-12 produced by peritoneal macrophages, revealing an early stage of maturation of these cells. APCs isolated from aa-fed mice were unable to stimulate a Th1 response in vitro. Both phenotypic features of T cells from aa-fed mice and their ability to produce a Th1 response in the presence of mature APCs were unaffected when compared with T cells from control mice. The results presented here support the notion that regulation of Th1 immunity to infection includes environmental factors such as dietary proteins, which provide a natural source of stimulation that contributes to the process of maturation of APCs.
Keywords: antigen-presenting cells, diet, immunological maturation, Leishmania
Introduction
Mucosal surfaces exceed the area of the skin by 100-fold and are the main surfaces in contact with external antigens. In humans, the area of the mucosal surface of the small intestine is estimated to be 300 m2. This large surface is constantly exposed to a variety of dietary antigens. It has been reported that 130–190 g of protein is absorbed daily,1 as intact, partially degraded or degraded molecules.2,3 The number of effector cells, such as plasm cells, in the small intestine is greater than that found in all other organs combined.4 Under normal conditions, the constant exposure to food proteins within this lymphoid tissue triggers local and systemic immunological activities, such as secretory immunoglobulin A (sIgA) production and oral tolerance.5 Indeed, lack of homeostasis of these activities can cause disorders such as food intolerance and allergies. Importantly, the beginning of central maturation of the immune system and its activities coincides with the weaning period, when a larger variety of food antigens encounter the mucosa of the small intestine. However, the selective importance of dietary antigens in the development of immunity has not been systematically addressed.
We previously reported that replacement of intact dietary proteins with equivalent amounts of amino acids (aa), in order to maintain nutritional requirements but disrupt the antigenic identity of dietary proteins, resulted in deficient development of the immune system. C57BL/6 mice fed this experimental diet from weaning to adulthood showed poor development of gut-associated lymphoid tissue, and low levels of sIgA and serum immunoglobulins. In vitro cytokine production by T cells from several lymphoid organs in response to non-specific stimuli showed a predominant immature T helper type 2 (Th2) profile, with high levels of interleukin (IL)-10 and IL-4, and low levels of interferon (IFN)-γ.6 These parameters resemble those found in suckling mice, suggesting that intake of antigenic proteins during the early stages of life is critical for the development of a Th1 profile. We also observed that mice reared on an aa-based diet developed decreased nasal tolerance to allergic asthma when compared with mice fed a control casein-containing diet,7 demonstrating the maintenance of this Th2 immature profile.
Experimental infection with the parasite Leishmania major has established the Th1/Th2 paradigm. C57BL/6 mice infected with L. major develop a Th1 response that results in IFN-γ production, macrophage activation, and control of parasite growth and lesions.8,9 In contrast, BALB/c mice respond to this parasite by developing a Th2 response that is associated with inefficient macrophage activation and poor control of parasite growth.8,10 Both dendritic cells (DCs) and macrophages are involved in the development and efficiency of the Th1 response against L. major in mice. DCs induce a primary response against the parasite during infection.11 DCs capture amastigotes and migrate to regional lymph nodes, where they become mature, up-regulating major histocompatibility complex (MHC) class II and costimulatory molecules, including CD80, CD86 and CD40. Mature DCs have a great ability to stimulate naïve CD4+ T cells to develop into Th1 cells.12,13 Macrophages capture the organisms at the site of inoculation and control intracellular pathogen growth under established Th1 immunity. Macrophages may either host or kill Leishmania, depending on the balance of the activities of inducible nitric oxide synthetase (iNOS) and arginase, which are competitively regulated by cytokines secreted by Th1 and Th2 cells. A Th1 immune response leads to the induction of iNOS, which results in production of nitric oxide (NO), which is essential for killing the parasite.14–16 In contrast, Th2 cytokines stimulate arginase production, which reduces killing capacity.17–19 Therefore, proper activation and maturation of antigen-presenting cells (APCs) resulting in Th1 immunity is fundamental to parasite control.
Based on the immature profile of the immune system of aa-fed C57BL/6 mice and the pronounced lack of a Th1 response to non-specific stimuli,6 we hypothesized that C57BL/6 mice reared on an aa-diet would exhibit deficient immunity against L. major. Such a result would demonstrate the importance of dietary antigenic proteins in promoting immunity against infections. Herein, we demonstrate that, indeed, dietary proteins seem to provide a natural source of stimulation, contributing to the function of APCs, which is important for the development of an efficient Th1 response against L. major.
Materials and methods
Mice
Inbred female C57BL/6 and BALB/c mice were bred and reared under conventional conditions in our facility. This study was approved by the local Ethical Committee for Animal Research (CETEA/UFMG/Brazil).
Diets
Mice were fed experimental diets containing either 15% casein or equivalent amounts of amino acids from weaning (3 weeks old) until adulthood (8 weeks of age).6 Diets (Rhoster Indústria e Comércio LTDA, SP, Brazil) were isocaloric and identical in terms of all other nutrients, according to the American Institute of Nutrition (AIN-93G).20 Tap water and diets in solid form as pellets were given ad libitum.
Evaluation of microbiota morphotypes
Fresh faeces, previously weighed, from mice fed either the casein diet or the aa diet was collected in sterile tubes. Decimal dilutions were then made in buffered saline. Aliquots of 0·1 ml were plated in non-selective blood agar. Plates were incubated at 37° for 48 hr under anaerobic conditions with CO2 gas. Bacteria were identified by macroscopic observation of the colonies and microscopic examination of cells with Gram staining.
Parasite, antigen and infection
Leishmania major (WHO MHOM/IL/80/Friedlin) was cultured in Grace’s insect medium (Gibco BRL, Grand Island, NY) supplemented with 20% heat-inactivated fetal calf serum (Cultilab, Campinas, Brazil), 2 mm l-glutamine (Sigma Chemical Co., St Louis, MO), 100 U/ml penicillin (Sigma Chemical Co.) and 100 μg/ml streptomycin (Sigma Chemical Co.) at 25°. Promastigotes were collected at the stationary phase (5th day of culture), centrifuged at 700 g at 4° for 15 min and washed two times in phosphate-buffered saline (PBS). Mice were infected with 1 × 106 parasites in 40 μl of PBS in the left hind footpad. Measurements of footpad thickness were taken using a caliper. The lesion size was calculated by subtracting the value for the uninfected contra lateral footpad from that of the infected footpad. Antigen was prepared from L. major promastigote stationary phase cultures as previously described.21 Briefly, cells were washed four times in PBS, the concentration was adjusted to 1 × 108 cells/ml, and cells were frozen/thawed three times. Antigen was homogenized and maintained at −20° until use.
Determination of parasite load
The number of living L. major parasites in infected tissues was determined by limiting dilution, as previously described.22 Briefly, single-cell suspensions from individual excised lesions were plated in log-fold serial dilutions in Grace’s insect tissue culture medium starting with a 1 : 10 dilution. Each sample was plated in quadruplicate and read microscopically 8 days after the beginning of the culture. Results were expressed as the mean of the negative log of the titre, i.e. the dilution corresponding to the last positive well.
Lymphocyte isolation and cell culture
Spleen and popliteal lymph node cell suspensions were prepared and adjusted to 5 × 106 cells/ml in RPMI-1640 (Gibco Laboratories) supplemented with 10% fetal bovine serum, 2 mm l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 25 mm HEPES, and 0·05 mm b-mercaptoethanol (Gibco Laboratories) and 1 ml was plated in 24-well tissue culture plates. Cells were stimulated in vitro with concanavalin A (ConA; 5 μg/ml; Sigma Chemical Co.) or 50 μl of L. major antigen. Supernatants were harvested at 24 hr for IL-12 and IL-10 and at 72 hr for IFN-γ, IL-4 and NO analysis. Isolation of lymphocyte and APC subpopulations was performed by incubation of the cells with monoclonal antibodies coupled to Microbeads (Dynal Biotech Inc., Oslo, Norway). Purified APCs and T cells from different groups of mice were stimulated in vitro with 50 μg of L. major antigen for 72 hr. Culture supernatants were harvested and analysed for IFN-γ production.
Cytokine assays and NO measurement
Levels of IL-12p70, IL-4, IL-10 and IFN-γ in supernatants were determined by sandwich enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s instructions (BD Bioscience, San Diego, CA). Briefly, supernatants were added to microtitre plates (Nunc, Rochester, NY), previously coated with rat anti-mouse IL-12, IL-4, IL-10 or IFN-γ monoclonal antibody (mAb) (BD Bioscience) at 1 ± 5 μg/ml and blocked with PBS containing 0·05% casein. Standards and samples were added and incubated overnight at 4°. Biotinylated rat anti-mouse mAb (BD Bioscience) was added, followed by peroxidase-labelled streptavidin (Sigma Chemical Co.). Bound cytokine was detected by the addition of H2O2 and o-phenylenediamine. Colour development was read at 495 nm after addition of 2 N H2SO4. Cytokine levels were calculated using a standard curve obtained with recombinant cytokines (BD Bioscience). The NO level in cell supernatants was determined using Griess reagent.23 This technique evaluates NO levels indirectly by correlation with the measurable levels of NO2−.
Analysis of cell subsets by flow cytometry
Cells were incubated with a combination of fluorescein isothiocyanate (FITC)-conjugated, phycoerythrin (PE)-conjugated, biotin-conjugated, allophycocyanin-conjugated or purified antibodies in the presence of 10% heat-inactivated normal rat serum at 4° for 30 min. Monoclonal antibodies used for staining were as follows: PE-labelled rat anti-mouse CD40, CD80 and CD86; streptavidin- and PE-labelled rat IgG2a; FITC-labelled rat IgG2a and rat anti-mouse CD11b; and cychrome-labelled rat anti-mouse CD11c (purchased from BD Bioscience). Stained cells were then applied to a FACScan (Becton Dickinson, Mountain View, CA). All data were analysed using Cellquest software (BD Bioscience, San Jose, CA, USA).
Analysis of IL-12p70 production by peritoneal macrophages
Peritoneal macrophages were isolated from the peritoneal cavities of mice injected 72 hr previously with 3% thyoglycolate. Macrophages were selected by submitting macrophage-enriched cell suspensions [5 × 106 cells/ml of Dulbecco’s modified Eagle’s minimal essential medium (DMEM) culture medium] to adhesion in 96-well plates for 4 hr at 37°, 5% CO2. Non-adherent cells and supernatants were harvested and plates washed three times with 200 μl/well of DMEM. Macrophages were cultured in triplicate in the presence of either DMEM (control) or lipopolysaccharide (LPS) (20 ng/well) plus IFN-γ (20 ng/well) for 24 hr at 37°, 5% CO2. The supernatants of the cultured macrophages were then collected for measurement of cytokine production. Concentrations of IL-12p70 in culture supernatants were measured using ELISA kits; the assays were performed according to the manufacturer’s instructions (R&D, Minneapolis, MN).
Statistical analysis
Results are expressed as the mean ± standard error of the mean (SEM). Normal distribution of our samples was confirmed by the Kolmogorov–Smirnov test. The significance of differences between groups was determined by Student’s t-test or analysis of variance (ANOVA) (Tukey’s post test). Means were considered statistically different when P < 0·05.
Results
C57BL/6 mice fed an amino acid-based diet are susceptible to L. major infection
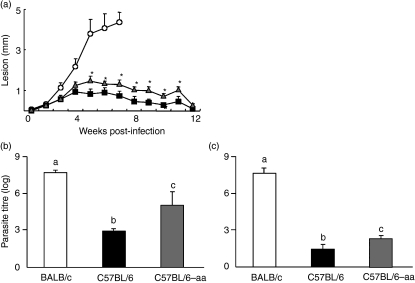
Because immunological maturation is required for the mounting of an efficient immune response to infections, we investigated the role of dietary protein in the development of immunity to an infectious parasite, L. major. C57BL/6 mice, a strain resistant to L. major infection, were fed an aa-based diet from weaning to 8 weeks of age, at which time they were inoculated in the hind footpad with 1 × 106 stationary-phase promastigotes. The course of infection was followed by measuring the development of primary lesions. As shown in Fig. 1a, aa-fed mice had reduced control of infection 5 weeks post-inoculation, showing increased footpad swelling as compared with control casein-fed C57BL/6 mice. However, we also observed that lesions in aa-fed C57BL/6 mice never reached the sizes found in the genetically susceptible BALB/c mice and showed a decline at 12 weeks post-infection, when lesion size became comparable to that in control casein-fed C57BL/6 mice (Fig. 1a). When numbers of lesion parasites were determined, C57BL/6 aa-fed mice were found to harbour significantly more parasites than C57BL/6 control mice and fewer parasites than BALB/c mice at 7 weeks of infection (Fig. 1b). Twelve weeks after infection, aa-fed C57BL/6 mice had a reduced parasite load, although this was not comparable to that of control mice (Fig. 1c). These results suggested that C57BL6 mice fed an aa diet were more susceptible to L. major infection than control mice, but that this susceptibility was not permanent as in the genetically susceptible BALB/c strain.
Figure 1.
Susceptibility to Leishmania major infection of amino acid (aa)-fed C57BL6 mice. (a) Casein-fed BALB/c (○), aa-fed C57BL/6 (Δ) or casein-fed C57BL/6 (▪) mice were infected with L. major promastigotas (1 × 106). Lesion size was monitored for 12 weeks and is expressed as the difference in thickness between the infected and the uninfected contralateral footpads. Numbers of lesion parasites at 7 (b) or 12 (c) weeks of infection were determined by limiting dilution assay and are expressed as the negative log10 dilution in casein-fed BALB/c mice (white bars), casein-fed C57BL/6 mice (black bars), and aa-fed C57BL/6 mice (grey bars) infected with L. major. Values are the mean ± standard error of the mean for five mice per group and are representative of results of two separate experiments. Asterisks or different letters show statistical difference between groups of C57BL/6 mice (P < 0·05).
C57BL/6 mice fed an aa-based diet and control mice have similar bacteria counts in the intestine
We previously reported that adult aa-fed mice exhibited an immature immune system.6 Herein, we found that the immaturity of aa-fed mice interfered with their resistance to L. major infection. Germ-free mice also show features of immunological immaturity such as poorly developed gut-associated lymphoid tissue (GALT), as well as low levels of secretory IgA and of serum IgG and IgA.24 Therefore, our first aim was to determine whether withdrawal of dietary proteins would affect the number of bacteria present in the intestinal microbiota. Table 1 shows that aa-fed mice and control mice had similar numbers of total morphotypes, and Gram-positive and Gram-negative bacteria in their intestinal content, suggesting that the drastic changes observed in aa-fed mice6 were not attributable to an alteration in the amount of intestinal microbiota.
Table 1.
Fecal bacteria counts from Cas-fed and aa-fed C57BL/6 mice
| Cas-diet | aa-diet | |
|---|---|---|
| Total morphotypes | 7·70 + 0·38 | 7·79 + 0·44 |
| Gram-positive bacteria | 6·06 + 0·16 | 6·92 + 0·77 |
| Gram-negative bacteria | 1·95 + 0·16 | 0·77 + 0·71 |
Feces from 8-week old C57BL/6 mice fed either Cas-diet or aa-diet since weaning were collected and plated in blood agar gel. Gram staining was used to distinguish Gram-positive and Gram-negative sub-types. Number of bacteria were counted as colony forming unities (CFU) and numbers represent log CFU/gram of feces.
Cas-fed mice = 10; aa-fed mice = 29.
Differences between groups were calculated by Student t-test, (P < 0·05).
C57BL/6 mice fed an aa-based diet develop reduced Th1 immunity to L. major
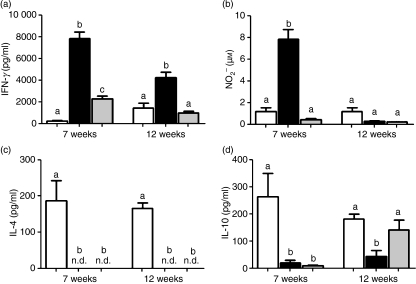
Because Th1 immunity is associated with resistance to L. major, and C57BL/6 aa-fed mice were previously found to have a decreased Th1 response in vitro to a non-specific stimulus, we next investigated the possibility that the increased susceptibility to L. major in this model is associated with a deficient Th1 response to the parasite. To test this, we determined IFN-γ production by lymphoid cells obtained from aa-fed and control mice 7 and 12 weeks after infection. Compared with controls, lymphoid cells from C57BL/6 aa-fed mice produced lower levels of IFN-γin vitro following stimulation with parasite antigen. Interestingly, this reduced level was maintained throughout the 12-week post-infection monitoring period (Fig. 2a). Indeed, the IFN-γ response to L. major antigen in aa-fed C57BL/6 mice was very similar to that found in BALB/c mice (Fig. 2a). Therefore, these results demonstrate a deficient Th1 response against L. major in aa-fed C57BL/6 mice.
Figure 2.
Amino acid (aa)-fed C57BL6 mice develop a weak T helper type 1 (Th1) response. Cell cultures from the spleen and popliteal lymph nodes of casein-fed BALB/c mice (white bars), casein-fed C57BL/6 mice (black bars), and aa-fed C57BL/6 mice (grey bars) at 7 and 12 weeks of infection with L. major were stimulated with L. major antigens. Supernatants were collected after 24 hr for interleukin (IL)-10 assays and after 72 hr for interferon (IFN)-γ, IL-4 and nitric oxide (NO) assays. The actual levels of cytokines and NO were calculated as the values for non-stimulated wells (medium alone) subtracted from the values obtained in stimulated cultures. All numbers represent the mean ± standard error of the mean for five mice per group and are representative of results of two separate experiments, Different letters show statistical difference between groups of C57BL/6 mice (P < 0·05).
Based on these results, we extended our analysis to evaluate the capacity of aa-fed C57BL/6 mice to produce NO2− (an indirect measurement of NO). As expected, aa-fed C57BL/6 mice produced reduced levels of synthesis in response to L. major at 7 weeks but not at 12 weeks post-infection (Fig. 2b). The lack of NO production would favour parasite growth, which might account for the high parasite load we found in aa-fed mice.
To test whether the decrease in the Th1 response was associated with a skewed immune response, the Th2 cytokine IL-4 was evaluated. Unlike lymphoid cells from BALB/c mice, which produce high levels of IL-4 in response to L. major, cells from aa-fed C57BL/6 mice secreted undetectable levels of IL-4, similar to the casein-fed C57BL/6 group (Fig. 2c). Thus, no apparent deviation towards a Th2 type response was detected in the increased susceptibility to L. major in aa-fed C57BL/6 mice. As IL-10 is a cytokine involved in down-modulating IFN-γ production and inflammatory reactions in infections with many parasites, including L. major, we analysed the production of this cytokine during infection. Although levels of IL-10 did not play an important role in the immune response of aa-fed C57BL/6 mice at 7 weeks after parasite inoculation, there was a significant increase in the levels of this cytokine 12 weeks after inoculation at levels comparable to those produced by BALB/c mice (Fig. 2d).
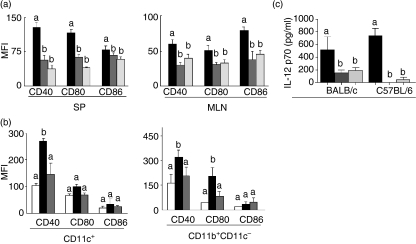
APCs from aa-fed C57BL/6 mice express low levels of the costimulatory molecules CD40 and CD80
It has been shown that a deficiency in the engagement between APCs and naïve T cells during L. major infection results in inefficient control of the parasite. We thus investigated the role of APCs in C57BL/6 aa-fed mice infected with L. major. As CD40, CD80 and CD86 molecules have been shown to be potent costimulatory molecules activating naïve T cells in response to L. major, we first compared expression of these molecules on APCs of naïve C57BL/6 aa-fed mice before infection with the profile of suckling mice to characterize their costimulatory maturity status. Flow cytometry analysis of these molecules in the gated CD11c+ population from the spleens and mesenteric lymph nodes of suckling and adult aa-fed mice showed very similar patterns of expression of these molecules, which significantly differed from those of adult mice on a casein diet (Fig. 3a). These results therefore suggest that dietary proteins are important for the process of maturation of APCs. We then performed the same analysis after infection. When expression of costimulatory molecules was analysed by flow cytometry in the gated CD11c+ (dendritic cell) and CD11b+ CD11c− (macrophage) populations from spleens of infected mice, we found that CD11b+ CD11c− cells from aa-fed C57BL/6 mice displayed reduced expression of the surface markers CD40 and CD80, similar to the pattern found on cells of BALB/c mice (Fig. 3b). Moreover, CD11c+ cells from aa-fed C57BL/6 mice expressed reduced levels of CD40 compared with the control C57BL/6 mice (Fig. 3b).
Figure 3.
Immature profile of antigen-presenting cells (APCs) from amino acid (aa)-fed C57BL6 mice. (a) Fluorescence-activated cell sorter (FACS) analysis for expression of CD40, CD80 and CD86 molecules in a CD11c gated population of lymphoid cells from the spleen (SP) or mesenteric lymph nodes (MLN) of control casein-fed C57BL/6 mice (black bars), control aa-fed C57BL/6 mice (dark grey bars) at 8 weeks of age and suckling mice at 3 weeks of age (light grey bars). (b) FACS analysis for expression of CD40, CD80 and CD86 molecules from splenic cells of casein-fed BALB/c mice (white bars), casein-fed C57BL/6 mice (black bars), and aa-fed C57BL/6 mice (grey bars) after infection with L. major. Macrophages are identified as CD11b+ CD11c− cells and dendritic cells are identified as CD11c+ cells. Values are mean fluorescence intensity (MFI) ± standard error of the mean (SEM) for five mice per group and are representative of results of two separate experiments. (b) Macrophages were isolated from the peritoneal cavitiy of control casein-fed (black bars), control aa-fed (dark grey bars) and suckling (light grey bars) BALB/c and C57BL/6 mice. They were stimulated in vitro with lipopolysaccharide (LPS) (20 ng/well) plus interferon (IFN)-γ (20 ng/well). Supernatants were collected 24 h later and interleukin (IL)-12p70 production was measured using an enzyme-linked immunosorbent assay (ELISA) kit. Values represent the mean ± SEM for five mice per group. Different letters show statistical difference between groups of C57BL/6 mice (P < 0·05).
To further investigate the state of maturation of macrophages of aa-fed mice, we tested the in vitro production of IL-12p70 by stimulated peritoneal macrophages isolated from BALB/c and C57BL/6 mice that were fed either a control diet (casein) or an aa diet. There was a significant reduction in IL-12p70 production by macrophages isolated from aa-fed mice (of both strains) as compared with those isolated from control casein-fed mice (Fig. 3c). This suggests that, in addition to their low expression of CD40 and CD80 during infection, macrophages from adult aa-fed naïve mice had features of functionally immature cells.
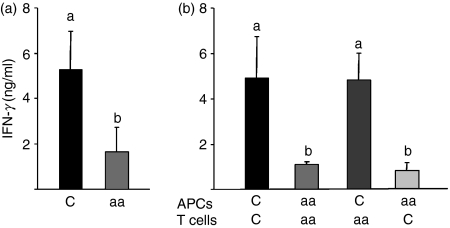
APCs from aa-fed mice stimulate a low Th1 response to L. major antigen
To investigate whether the immaturity of APCs from C57BL/6 aa-fed mice was related to the low Th1 response observed upon infection in these mice (Fig. 4a), we analysed the capacity of APCs from C57BL/6 aa-fed mice to stimulate T cells in vitro upon stimulation with L. major antigen. After infection with L. major, T cells and APCs were purified from aa-fed and control C57BL/6 mice using magnetic beads, and the isolated populations were stimulated at different combinations in vitro with L. major antigen. We used a ratio of 1 : 5 for all APC:T-cell combinations. As expected, production of IFN-γ by isolated populations from the same donor was consistent with the results obtained for total populations (Fig. 4a). However, T cells from aa-fed mice when activated with APCs from control mice recovered their capacity to produce IFN-γ in response to L. major antigen. Moreover, T cells from control mice, which were able to produce large amounts of IFN-γ upon activation with control APCs, failed to produce IFN-γ in the presence of APCs from aa-fed mice (Fig. 4b). Therefore, our results suggest that dietary proteins provide a natural source of stimulation important for the process of up-regulation of costimulatory molecules on APCs, which is an important mechanism for Th1 immunity to infectious agents, such as L. major.
Figure 4.
Antigen-presenting cells (APCs) from C57BL/6 amino acid (aa)-fed mice are unable to induce a T helper type 1 (Th1) response to Leishmania major antigen. Lymphocytes and APC populations from aa-fed mice and the control group were isolated by incubation with monoclonal antibodies coupled to microbeads. (a) Spleen cells from casein-fed (C) and aa-fed mice and (b) different combinations of purified APCs and T cells, at a ratio 1 : 5, from different groups of mice (b) were stimulated in vitro with 50 μg of L. major antigen for 72 hr. Culture supernatants were harvested and analysed for interferon (IFN)-γ production by enzyme-linked immunosorbent assay (ELISA). Values are the mean ± standard error of the mean for five mice per group and are representative of results of two separate experiments. Different letters show statistical difference between groups (P < 0·05).
Discussion
In the present study, we showed that food antigens are important in early life for the development of immunity against L. major. We found that aa-fed C57BL/6 mice, which are genetically resistant to L. major infection, exhibited enhanced footpad lesions and high numbers of parasites. We also found lower levels of IFN-γ and NO, suggesting that the capacity of these mice to kill the parasite was also impaired. Analysis of APCs (dendritic cells and macrophages) of aa-fed mice revealed deficient levels of costimulatory molecules (CD40 and CD80), reduced production of IL-12p70 by macrophages and low capacity of APCs to stimulate a T-cell response. This profile, which is consistent with an immature phenotype of APCs, is associated with a deficient capacity to mount a Th1 immune response to L. major. Analysis of the amount of microbiota in the intestinal contents of aa-fed mice showed no alteration, suggesting that there is no change in intestinal bacteria numbers that may account for the immaturity of aa-fed mice. Our results suggest that natural antigenic stimulation in the gut and subsequent host immunity are intertwined, determining activation of immune pathways that eventually lead to positive regulation of the host immune response.
Leishmaniasis is a disease that is endemic in underdeveloped countries. Immunological and epidemiological studies suggest that effective immunity in hosts can be developed. However, the high prevalence in these populations is a result not only of genetic but also of environmental factors. Two environmental factors are nutritional inadequacies and onset of infection in early life.25 Perhaps, in these two situations, the immaturity of the immune system may play a role.
In the light of the discovery of the Th1/Th2 paradigm in leishmaniasis, it is clear that genetically resistant mouse strains are able to respond to L. major through Th1 immunity, but strains that are unable to mount a Th1 response against the parasite are susceptible to the infection.26 Interestingly, although the increased susceptibility to L. major observed in our aa-fed mouse model was associated with an impaired Th1 response, Th1/Th2 deregulation, which appears to be the mechanism involved in the development of lesions in the genetically susceptible BALB/c strain, was not observed. T cells from aa-fed C57BL/6 mice showed a reduction in the levels of IFN-γ but no change in IL-4 production. Therefore, our data broaden the sources of regulation of Th1 immunity beyond genetic factors to include environmental factors, such as dietary proteins, which appear to enhance Th1 immunity against infections.
In addition, we observed that aa-fed C57BL/6 mice were more susceptible to L. major infection and presented lesions that were larger than those found in casein-fed C57BL/6 mice. Nevertheless, these lesions never reached the size of lesions displayed by BALB/c mice, and a clear reduction to lesion sizes comparable to those of casein-fed C57BL/6 mice were found at 12 weeks post-infection. Lesions associated with L. major infection are composed of parasites and inflammatory cells that are recruited to the site of infection. We found that a decrease in the lesion sizes of aa-fed C57BL/6 mice 12 weeks after infection was associated with a reduction in the parasite load. In addition, the high levels of IL-10 at this time-point might also play a role in the reduction of lesion size observed in aa-fed C57BL/6 mice by immunomodulating the recruitment and local proliferation of inflammatory cells. Therefore, the susceptibility to L. major infection of aa-fed C57BL/6 mice was transitory. Indeed, we have shown previously that the immature state of adult aa-fed C57BL/6 mice is not permanent and immunological maturity can be restored by feeding a diet containing 10% protein for a period as short as 72 hr.27
The immature immunological state of aa-fed mice is similar to that reported for germ-free mice. These animals also show poorly developed GALT, and low levels of secretory IgA and serum IgG and IgA.24 Analysis of the total number of Gram-positive and Gram-negative bacteria in the intestinal contents of aa-fed C57BL6 showed no alteration, suggesting that replacement of proteins by amino acids does not result in a reduction of intestinal microbiota. A recent study on the effect of elemental diet (ED) in a colitis model using CB-17 severe combined immunodeficiency (SCID) mice into which IL-10−/− cells were transferred showed that ED-fed mice have reduced total amounts of faecal bacteria.28 Our data on aa-fed mice may not be comparable to the results obtained in animals fed an ED. The experimental diet we used had carbohydrates, lipids and all other components in their native form; only proteins were replaced by amino acids. The possibility remains that the aa diet exerts effects on the specific microbiota component that colonizes the gut. This possibility is currently being tested. However, the drastic arrest in immunological maturation presented by aa-fed mice6 is only comparable to that found in germ-free animals and it is unlikely that more subtle alterations in microbiota would yield similar results.
The mechanisms underlying Th1 differentiation are complex and involve accessory cells and molecules. The contribution of mature APCs in Th1 differentiation is evident. Immature APCs such as found in neonates show little or no increase in expression of costimulatory markers such as CD40, CD80 and human leucocyte antigen (HLA)-DR. These cells produce low levels of pro-inflammatory cytokines such as IL-12 and are unable to induce Th1 immunity.29–31 Other studies also showed that dendritic cells from neonates exhibited diminished endocytotic activity as well as decreased capacity to stimulate T cells.32–34 However, it has been demonstrated that, under optimal costimulatory conditions or with allogenic dendritic cells, neonatal and adult T-cell responses are similar, showing the immunocompetence of the T cells.34,35 The increased susceptibility of neonates to infections in general has been ascribed to the immaturity of their immune system, and, more specifically, to the particular conditions of antigen presentation and T-cell priming.36,37 In agreement with these results, we found that APCs isolated from the spleens and mesenteric lymph nodes of aa-fed adult C57BL/6 mice expressed CD40, CD80 and CD86 at levels similar to those of APCs from suckling mice. CD40 and CD80 expression were also decreased on dendritic cells and macrophages from aa-fed C57BL/6 mice infected with L. major.
It has been well demonstrated that dendritic cells up-regulate MHC class I and II, and CD86 and CD40 surface molecules after ingestion of promastigotes of L. major in genetically resistant mouse strains, indicating that maturation of these cells is a critical mechanism involved in the resistance to the parasite. Susceptibility to L. major infection has been linked to a deficiency in the engagement between CD40 and CD40L.8,38–44 Furthermore, B7-mediated costimulation is required for the development of the early immune response following infection of either resistant or susceptible mice.45 Therefore, activation and maturation of APCs with expression of MHC molecules, CD40 and CD80 on the surface of APCs is fundamental to the process of costimulation of T cells for an efficient response to L. major infection.
After endocytosis, APCs from resistant mice produce pro-inflammatory cytokines such as IL-12. IL-12 is a cytokine that is determinant in the CD4+ T-cell differentiation to Th1.46 Infected macrophages of C57BL/6 mice produce high levels of IL-12 and are able to drive Th1 immunity. However, it has been reported previously that APCs from neonates are deficient in pro-inflammatory cytokine secretion.47 Consistent with the results for costimulatory molecule expression, production of IL-12p70 by peritoneal macrophages of pre-weaning and aa-fed C57BL/6 and BALB/c mice was significantly reduced compared with macrophages from control casein-fed mice. As important APCs and target cells in L. major infection, macrophages may have a critical role in determining the activation of effective T-cell immunity.
As antigens are captured and processed, up-regulation of costimulatory molecules and cytokine production takes place to promote activation of T cells. By culturing isolated T cells and APCs, we found that APCs from C57BL/6 aa-fed mice were unable to induce Th1 polarization in response to L. major antigen, regardless of the source of T lymphocytes. Our results indicate that APCs from aa-fed mice infected with L. major were deficient in the process of maturation towards efficient Th1 inducers, presumably as a consequence of the lack of external21 stimulation normally provided by dietary protein antigens during development.
To date, no data have shown that dietary antigens are important for the development of immunocompetence and infection resistance. There have been studies reporting a decrease in the immune response of protein-energy malnourished BALB/c mice to Leishmania chagasi vaccination.48 Studies from other groups confirm the influence of protein malnutrition on effective immune responses to Leishmania infection.49 These authors used a diet with as little as 3% protein content, and the animals showed all the signs of malnutrition. In our model, dietary proteins were not simply removed or reduced; they were replaced by equivalent amounts of amino acids. The nutritional state of aa-fed animals was found to be normal when determined using several nutritional parameters (serum protein and albumin levels, blood cell count, fur appearance, and behaviour; data not shown).6 Therefore, we ascribed the changes we found to a direct stimulatory effect of antigenic dietary proteins in the immune system rather than an indirect impact of malnutrition.
Nevertheless, it is tempting to speculate that a similar immature profile of APCs might be present in immature immune states, such as in young children and in protein malnutrition states, in which environmental factors account for the increased susceptibility to L. major. The stimulatory effects of food proteins in APC maturation and T-cell activation may further demonstrate the critical role of a well-balanced diet not only for general health but also for the proper development of immunity.
Acknowledgments
This work was supported by CNPq and FAPEMIG grants and research fellowships from CNPq (to AMCF and LQV) and CAPES (to JSM and JFA), Brazil. We thank Ilda Marçal de Souza for animal care; Frankcinéia A. Assis for technical assistance; and Eric Milner for editorial assistance.
Disclosures
None of the authors have any potencial financial conflict of interest related to this work.
References
- 1.Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56(1 Pt 2):S5–18. doi: 10.1111/j.1753-4887.1998.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 2.Husby S, Jensenius JC, Svehag SE. Passage of undergraded dietary antigen into the blood of healthy adults. Scand J Immunol. 1985;22:83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 3.Bruce MG, Strobel S, Hanson DG, Ferguson A. Irradiated mice lose the capacity to ‘process’ fed antigens for systemic tolerance of delayed hypersensitivity. Clin exp Immunol. 1987;70:611–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–76. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 5.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes Jda S, Mucida Dde S, Cara DC, Alvarez-Leite JI, Russo M, Vaz NM, de Faria AM. Stimulation by food proteins plays a critical role in the maturation of the immune system. Int Immunol. 2003;15:447–55. doi: 10.1093/intimm/dxg043. [DOI] [PubMed] [Google Scholar]
- 7.Mucida DS, Rodriguez D, Keller AC, Gomes E, Menezes JS, de Faria AM, Russo M. Decreased nasal tolerance to allergic asthma in mice fed an amino acid-based protein-free diet. Ann N Y Acad Sci. 2004;1029:361–5. doi: 10.1196/annals.1309.042. [DOI] [PubMed] [Google Scholar]
- 8.Heinzel FP, Rerko RM, Hujer AM. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998;184:129–42. doi: 10.1006/cimm.1998.1267. [DOI] [PubMed] [Google Scholar]
- 9.Belosevic M, Finbloom DS, Van Der Meide PH, Slayter MV, Nacy CA. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J Immunol. 1989;143:266–74. [PubMed] [Google Scholar]
- 10.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990;171:115–27. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moll H. Dendritic cells and host resistance to infection. Cell Microbiol. 2003;5:493–500. doi: 10.1046/j.1462-5822.2003.00291.x. [DOI] [PubMed] [Google Scholar]
- 12.Moll H. Epidermal Langerhans cells are critical for immunoregulation of cutaneous leishmaniasis. Immunol Today. 1993;14:383–7. doi: 10.1016/0167-5699(93)90138-B. [DOI] [PubMed] [Google Scholar]
- 13.von Stebut E, Belkaid Y, Jakob T, Sacks DL, Udey MC. Uptake of Leishmania major amastigotes results in activation and interleukin 12 release from murine skin-derived dendritic cells: implications for the initiation of anti-Leishmania immunity. J Exp Med. 1998;188:1547–52. doi: 10.1084/jem.188.8.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–16. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 15.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–7. [PubMed] [Google Scholar]
- 16.Stenger S, Thuring H, Rollinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. J Exp Med. 1994;180:783–93. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goerdt S, Orfanos CE. Other functions, other genes: alternative activation of antigen-presenting cells. Immunity. 1999;10:137–42. doi: 10.1016/s1074-7613(00)80014-x. [DOI] [PubMed] [Google Scholar]
- 18.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 19.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–54. [PubMed] [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Hodes RJ. Molecular alterations in the aging immune system. J Exp Med. 1995;182:1–3. doi: 10.1084/jem.182.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira LQ, Goldschmidt M, Nashleanas M, Pfeffer K, Mak T, Scott P. Mice lacking the TNF receptor p55 fail to resolve lesions caused by infection with Leishmania major, but control parasite replication. J Immunol. 1996;157:827–35. [PubMed] [Google Scholar]
- 23.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;141:2407–12. [PubMed] [Google Scholar]
- 24.Bos NA, Meeuwsen CG, Wostmann BS, Pleasants JR, Benner R. The influence of exogenous antigenic stimulation on the specificity repertoire of background immunoglobulin-secreting cells of different isotypes. Cell Immunol. 1988;112:371–80. doi: 10.1016/0008-8749(88)90306-1. [DOI] [PubMed] [Google Scholar]
- 25.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–18. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amaral JF, Foschetti DA, Assis FA, Menezes JS, Vaz NM, Faria AM. Immunoglobulin production is impaired in protein-deprived mice and can be restored by dietary protein supplementation. Braz J Med Biol Res. 2006;39:1581–6. doi: 10.1590/s0100-879x2006001200009. [DOI] [PubMed] [Google Scholar]
- 28.Kajiura T, Takeda T, Sakata S, Sakamoto M, Hashimoto M, Suzuki H, Suzuki M, Benno Y. Change of intestinal microbiota with elemental diet and its impact on therapeutic effects in a murine model of chronic colitis. Dig Dis Sci. 2009;54:1892–900. doi: 10.1007/s10620-008-0574-6. [DOI] [PubMed] [Google Scholar]
- 29.Orlikowsky TW, Spring B, Dannecker GE, Niethammer D, Poets CF, Hoffmann MK. Expression and regulation of B7 family molecules on macrophages (MPhi) in preterm and term neonatal cord blood and peripheral blood of adults. Cytometry. 2003;53:40–7. doi: 10.1002/cyto.b.10033. [DOI] [PubMed] [Google Scholar]
- 30.Simpson CC, Woods GM, Muller HK. Impaired CD40-signalling in Langerhans’ cells from murine neonatal draining lymph nodes: implications for neonatally induced cutaneous tolerance. Clin Exp Immunol. 2003;132:201–8. doi: 10.1046/j.1365-2249.2003.02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langrish CL, Buddle JC, Thrasher AJ, Goldblatt D. Neonatal dendritic cells are intrinsically biased against Th-1 immune responses. Clin Exp Immunol. 2002;128:118–23. doi: 10.1046/j.1365-2249.2002.01817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elbe A, Tschachler E, Steiner G, Binder A, Wolff K, Stingl G. Maturational steps of bone marrow-derived dendritic murine epidermal cells. Phenotypic and functional studies on Langerhans cells and Thy-1+ dendritic epidermal cells in the perinatal period. J Immunol. 1989;143:2431–8. [PubMed] [Google Scholar]
- 33.Muthukkumar S, Goldstein J, Stein KE. The ability of B cells and dendritic cells to present antigen increases during ontogeny. J Immunol. 2000;165:4803–13. doi: 10.4049/jimmunol.165.9.4803. [DOI] [PubMed] [Google Scholar]
- 34.Delespesse G, Yang LP, Ohshima Y, Demeure C, Shu U, Byun DG, Sarfati M. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine. 1998;16:1415–9. doi: 10.1016/s0264-410x(98)00101-7. [DOI] [PubMed] [Google Scholar]
- 35.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science (New York, NY) 1996;271:1723–6. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 36.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 37.Chang-Rodriguez S, Ecker R, Stingl G, Elbe-Burger A. Autocrine IL-10 partially prevents differentiation of neonatal dendritic epidermal leukocytes into Langerhans cells. J Leukoc Biol. 2004;76:657–66. doi: 10.1189/jlb.0204087. [DOI] [PubMed] [Google Scholar]
- 38.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–81. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 39.Field AE, Wagage S, Conrad SM, Mosser DM. Reduced pathology following infection with transgenic Leishmania major expressing murine CD40 ligand. Infect Immun. 2007;75:3140–9. doi: 10.1128/IAI.00160-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa C. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 41.Padigel UM, Perrin PJ, Farrell JP. The development of a Th1-type response and resistance to Leishmania major infection in the absence of CD40-CD40L costimulation. J Immunol. 2001;167:5874–9. doi: 10.4049/jimmunol.167.10.5874. [DOI] [PubMed] [Google Scholar]
- 42.Marovich MA, McDowell MA, Thomas EK, Nutman TB. IL-12p70 production by Leishmania major-harboring human dendritic cells is a CD40/CD40 ligand-dependent process. J Immunol. 2000;164:5858–65. doi: 10.4049/jimmunol.164.11.5858. [DOI] [PubMed] [Google Scholar]
- 43.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–9. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 44.Soong L, Xu JC, Grewal IS, et al. Disruption of CD40–CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–73. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 45.Elloso MM, Scott P. Expression and contribution of B7-1 (CD80) and B7-2 (CD86) in the early immune response to Leishmania major infection. J Immunol. 1999;162:6708–15. [PubMed] [Google Scholar]
- 46.Schmitt E, Hoehn P, Germann T, Rude E. Differential effects of interleukin-12 on the development of naive mouse CD4+ T cells. Eur J Immunol. 1994;24:343–7. doi: 10.1002/eji.1830240211. [DOI] [PubMed] [Google Scholar]
- 47.Lee HH, Hoeman CM, Hardaway JC, et al. Delayed maturation of an IL-12-producing dendritic cell subset explains the early Th2 bias in neonatal immunity. J Exp Med. 2008;205:2269–80. doi: 10.1084/jem.20071371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malafaia G, Serafim TD, Silva ME, Pedrosa ML, Rezende SA. Protein-energy malnutrition decreases immune response to Leishmania chagasi vaccine in BALB/c mice. Parasite Immunol. 2009;31:41–9. doi: 10.1111/j.1365-3024.2008.01069.x. [DOI] [PubMed] [Google Scholar]
- 49.Perez H, De La Rosa M, Malave I. The effect of protein restriction on the development of protective immunity to Leishmania mexicana. Parasite Immunol. 1984;6:285–94. doi: 10.1111/j.1365-3024.1984.tb00801.x. [DOI] [PubMed] [Google Scholar]