Abstract
Problem statement
The development of a potent vaccine that can help treat tumors resistant to conventional cytotoxic therapies remains elusive. While part of the problem may be that trials have focused on patients with bulky residual disease, the desire to maximize responses to the vaccine remains.
Approach
The gamma(c) family of cytokines offer a unique opportunity to support the expansion and effector potential of vaccine-responding T-cells, as well as stimulate other effectors, such as natural killer (NK) cells, to become activated.
Results
Combining vaccines with cytokines seems logical but can bring unwanted toxicity, as has been observed with interleukin (IL)-2. In addition, the nonspecific activation or expansion of unwanted cell subsets, such as regulatory T-cells, can contribute to global immunosuppression and limit vaccine responses. The development of IL-7 and IL-21 for the clinic offers the promise of enhancing anti-tumor responses but with far less systemic toxicity and no expansion of regulatory T cells. Preclinical studies demonstrate that IL-15 could also improve T-cell, and especially NK-cell, responses as well.
Conclusions/Recommendations
Future work should expand the use of vaccines with IL-7, IL-21 and hopefully IL-15 in high-risk patients, and consider treatment while in a state of minimal residual disease to maximize benefit. Identifying tumors that can signal through gamma(c) cytokines will also be essential so that induction of relapse will be avoided.
Keywords: Interleukin-2, interleukin-7, interleukin-15, interleukin-21, tumor vaccines
Introduction
Despite the success of multimodality therapy for cancer including chemotherapeutic agents, radiation, and surgery, relapse still occurs in a large percentage of patients. Immune effector cells represent a powerful tool for eliminating residual tumor cells. The potency of donor lymphocyte infusions (DLI) in treating chronic myelogenous leukemia following hematopoietic stem cell transplant (HSCT) is a striking example of the potential of cancer immunotherapy. Clinical responses to adoptive cell therapies administered in the autologous setting further illustrate the promise of cancer immunotherapy. The development of vaccines designed to elicit adaptive immune responses, mainly from T-cells, has occurred in parallel with adoptive cell therapy, but it is evident based on the clinical data generated with vaccines thus far that approaches to increase potency will be necessary. Indeed, the great appeal of this approach is the potential to generate immunotherapies that are antigen-specific, with a particular focus on tumor-associated antigens. One of the main drawbacks to this approach, however, is that tumor antigens represent self-proteins, which can induce tolerance in the host. Thus, vaccines have been examined in combination with other immunotherapies as a means of generating a potent T-cell response against tumor antigens while overcoming the barriers of tolerance.
Vaccines are being pursued for multiple types of cancers and are being designed using multiple approaches to enhance immunogenicity. Administration of whole cancer cells, purified peptides, or DNA vaccines have been given alone, or in combination, with professional antigen presenting cells (APCs) to elicit immunogenic responses. Combinations of different types of immunotherapy with traditional modalities like chemotherapy, radiation and surgery, have been tested in clinical trials with some promise. Cytokines delivered with vaccines as adjuvants aim to improve antitumor immunity by increasing the proliferation of effector cells, and also improving their cytotoxicity or cytokine production1. It has been demonstrated in preclinical models that concurrent administration of cytokines with a vaccine has the potential to enhance immune reactivity through the recruitment of T cells and APCs to lymphoid organs, as well as by activation of T-cells and natural killer (NK) cells directly. In this review, we will discuss clinically relevant cytokines that have been coupled with a vaccine or cellular therapy in preclinical models, and in some cases clinical trials, to generate antitumor immune responses, with a focus on the so-called gamma(c) family of cytokines. These cytokines (Interleukin 2, Interleukin 7, Interleukin 15, and Interleukin 21) all utilize the common cytokine gamma chain for signaling and have potent effects on T-cells and NK cells, the major effectors in the anti-tumor immune response.
Interleukin 2
Background
The first cytokine administered in a vaccine trial against cancer was interleukin 2 (IL-2). IL-2 is produced mainly by T helper cells, acts on a variety of immune cells across the innate and adaptive immune system and is known to play an important role in the initiation and maintenance of antigen-specific immune responses (Figure 1). The biology and signaling pathways of IL-2 have been reviewed extensively1,2. Because of the broad effects of IL-2 on a variety of immune cells, the specific mechanisms by which IL-2 influences the immune system to induce tumor regression are not completely understood.
Figure 1. Target lymphocyte populations of gamma(c) cytokines.
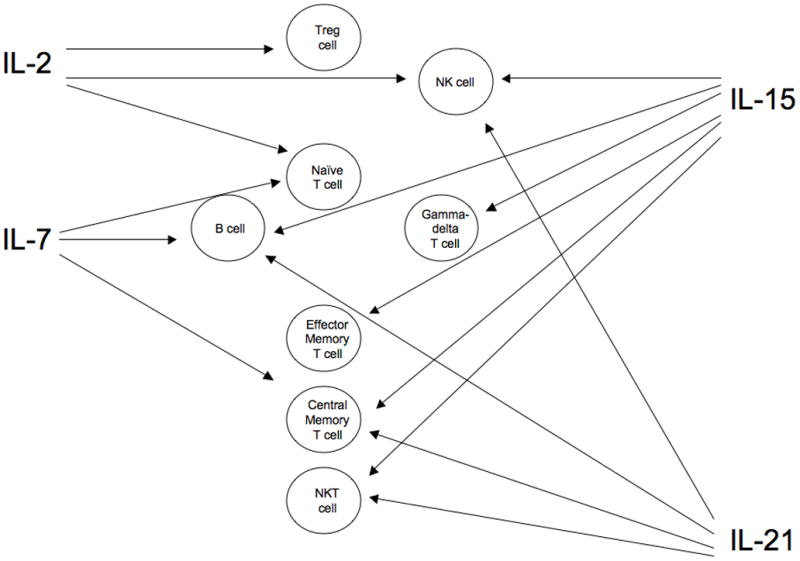
While there is considerable overlap of cytokine activity on lymphocytes, effects can be stimulatory or inhibitory depending on the cytokine. IL-21 only increases proliferation of T cells stimulated with anti-CD3 or antigen, but can augment responses to other gamma(c) cytokines. IL-7 acts on developing B cells but not mature cells of this lineage. IL = interleukin, NK = natural killer, NKT = natural killer-T cell, Treg = regulatory T cell
A variety of preclinical models demonstrated potential therapeutic benefit of combining IL-2 with vaccines, leading to the study of IL-2 in clinical trials. The antitumor effect of IL-2 is believed to be mediated by lymphocyte expansion and augmentation of effector cell function2. However, while IL-2 enhances the activity of both NK cells and T-cells, it can also expand regulatory T-cells (Tregs), which contribute to tumor-associated immunosuppression3. The Food and Drug Administration approved IL-2 as a single agent for use in patients with metastatic renal-cell carcinoma (RCC) (1992) and metastatic melanoma (1998). IL-2 has also shown efficacy as an adjuvant for infectious vaccine therapy. As will be outlined below, objective tumor responses have been observed in clinical trials combining cancer vaccines with IL-2. An informative commentary on the history of IL-2 treatment for melanoma is available4. This review will explore IL-2 as a prototypic cytokine that acts as an adjuvant to vaccine therapy by examining the relevant clinical trials in melanoma, where there is the most clinical experience in adults, as well as in pediatric solid tumors. A review of the experience with IL-2 and vaccines in RCC is discussed elsewhere5. Given the diversity of clinical trial designs using IL-2 as an adjuvant, we will also discuss dosing, timing of administration and reported objective response rates as potential factors that may impact efficacy.
Clinical Trials with Vaccines and IL-2 in Melanoma
When examining the literature on IL-2 given with therapeutic vaccines for melanoma, studies vary by the type of vaccine administered, IL-2 dose, timing of IL-2 administration in relation to the vaccine, and length of therapy (Table 1). The earliest data for systemic IL-2 following vaccination was reported by Rosenberg et al. in 1998, who used high-dose IL-2 immediately following vaccination with a modified gp100 (a relevant melanoma tumor-associated antigen) peptide vaccine in 31 patients with melanoma. A 42% objective response rate was reported, while the vaccine alone had a 5% response rate6. This initial observation led to the development of further trials with more advanced vaccines, but served as a “proof of principle” study that vaccines combined with a cytokine can improve objective tumor response rates over vaccines alone. Another report by this group with a different peptide vaccine and IL-2 demonstrated a 38% objective response rate, although it was not documented whether they were partial remissions (PRs) or complete remissions (CRs), nor how long responses were maintained7. However, other trials in melanoma incorporating peptide vaccines with IL-2 have shown either response rates similar to that seen with IL-2 alone (about 15–25%), or no responses at all8,9. One trial did not report a specific response rate10.
Table 1.
Analysis of melanoma trials where patients received both IL-2 and a vaccine
| Vaccine | IL-2 Dose/route | Timing/length of IL-2 therapy | Objective response rate | Ref. |
|---|---|---|---|---|
| Peptide (Day+0) | ||||
| g209-2M | 720,000IU/kg I.V. | Start Day+1 or +5 until grade 3–4 irreversible toxicity (tolerance) | 42% | [6] |
| g209-2M | 720,000IU/kg I.V. | Same as above (6–10 doses) | 38% | [7] |
| Tyrosinase+ gp100+ tetanus helper | 3,600,000IU/m2 S.C. | Group 1: Start Day+7 Group 2: Start Day+28. Daily for 6 weeks in both groups |
Not reported | [10] |
| g209-2M | 5,000,000IU/m2 S.C. | Days +0 to +4 and days +7 to +12 repeated every 21 days | 0% | [8] |
| g209-2M | 600,000IU/kg I.V. | Start Day+1 for 5 days on: Weeks 1 and 3 for trial one Weeks 7 and 9 for trial two Weeks 1, 4, 7 & 10 for trial three |
16% | [9] |
| DC or PBMCs | ||||
| DC+MART-1+ g209-2M | 720,000IU/kg I.V. | Start Day+0 for 3 days | 0% | [12] |
| DC+tumor lysate | 700,000IU S.C. | Start Day+0, 3 times/week | 0% | [14] |
| Tumor/DC Hybrid Cell | 3,000,000IU S.C. | Start Day+0 for 6 days | 0% | [15] |
| DC+tumor lysate | 2,400,000IU/m2 S.C. | Start Day+1 for 3 days | 0% | [16] |
| Tumor/DC Hybrid Cell | 3,000,000IU/m2 | Start Day+1 for 5 days | 10% | [17] |
| PBMC+g209-2M | 720,000IU/kg I.V. | Start Day+0 until tolerance (4–11 doses) | 0% | [18] |
| DC+tumor lysate | 3,000,000IU S.C. or 360,000IU/kg I.V. | Low: Start Day+0 for 4 days High: Start Day+0 for 9 doses |
0% | [13] |
| DC+tumor lysate or DC+peptide cocktail | 1,000,000IU/m2 S.C. | Start Day+1 for 5–14 days | 0% (lysate) 22% (peptides) |
[11] |
| Other | ||||
| Adenovirus+MART-1 or Adenovirus+gp100 | 720,000IU/kg I.V. | Start Day+1 until tolerance | 16% | [20] |
| Fowlpox virus+g209-2M | 720,000IU/kg I.V. | Start Day+0 up to 12 doses every 4 weeks | 50% | [21] |
| SRL172 (Mycobacterium) | 6,000,000IU S.C. | Start Day+0 for 3 days | 19% | [23] |
| DNP+BCG+tumor | 3,000,000IU S.C. or 720,000IU/kg I.V. | Low: Start Day+0 for 5 days every 14–21 days High: Start Day+0 for 2 weeks, one week rest, then repeat once |
42% | [19] |
| Tumor plasma membrane on silica beads | 1,750,000IU/m2 S.C. | Start Day+5 from vaccine for 1 week | 0% | [22] |
I.V. = intravenous, S.C. = subcutaneous
While it could be argued that peptides are inefficient as vaccines since they need to be expressed by an APC and presented with costimulatory signals to get a proper effector response, studies have also examined IL-2 given with a dendritic cell (DC) vaccine. In these studies, the vaccine is potentially capable of presenting a melanoma antigen directly, and IL-2 could facilitate expansion of any vaccine-responding cells. Unfortunately the response rates in these trials were quite poor11–17. One group even adopted a similar approach using peripheral blood mononuclear cells (PBMC), instead of DCs to patients pretreated with other vaccines, and saw no objective clinical responses18.
In contrast to using APCs, one report using a dinitrophenyl-modified autologous melanoma cell vaccine as either a primary treatment or as an adjuvant showed 42% objective tumor regression (2CR, 8PR) following combination with IL-2, lasting for a median duration of 6 months (range 3–50 months)19. Viruses have also been explored as a means of presenting overexpressed melanoma antigens, and whereas adenovirus vaccines yielded no responses20, another report using a recombinant fowlpox virus encoding a minigene construct encoding a single, modified melanoma epitope yielded a 50% response rate (3CR, 3PR) when given with IL-221. Regarding nonviral approaches, using melanoma plasma membranes on silica beads showed no responses22, and giving only heat-killed Mycobacterium without any tumor antigens was no better than giving IL-2 alone23. Thus, no controlled randomized vaccine trial ± IL-2 have reported that IL-2 improves responses to a tumor vaccine, and in a multitude of non-controlled trials response rates to tumor vaccines remain low, whether or not IL-2 is co-administered. Moreover, a meta-analysis of the vaccine trials at the National Cancer Institute (NCI) demonstrated that melanoma vaccines in general, when given with IL-2, do no better than giving IL-2 alone24.
Clinical Trials with Pediatric Cancers
The clinical experience in pediatrics with IL-2 and vaccines has been more limited with neuroblastoma the most extensively studied. Investigators have used IL-2-secreting autologous neuroblastoma cell lines as vaccines, and a transgenic chemokine-cytokine (lymphotactin-IL-2) vaccine generated in autologous and allogeneic neuroblastoma cell lines25–27. Except for a few patients with PRs or CRs, all of the response rates were disappointing. Importantly, very recent work has demonstrated that IL-2 combined with GM-CSF and a monoclonal antibody against GD2-expressing neuroblastomas can lead to enhanced event-free and overall survival in a phase III trial28. This data demonstrates the efficacy of IL-2 as an adjuvant to monoclonal antibody therapy rather than as an adjuvant for T-cell active vaccines, and illustrates the potential effectiveness of cytokines in cancer immunotherapy in the context of a large, well-designed clinical trial conducted at multiple centers through a cooperative oncology group.
In addition to neuroblastoma, Dagher et al. reported in 2002 no clinical benefit of a PBMC vaccine pulsed with tumor peptides given with IL-2 in children with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma29. A followup study by Mackall et al. in the same patient populations gave autologous T-cells and DCs pulsed with peptides derived from tumor-specific translocation breakpoints, and was reported in 2008 using different doses of IL-2 administered to three cohorts30. Immune responses to the translocation breakpoint peptides occurred in only 39% of patients. There was a 43% 5-year overall survival for patients initiating immunotherapy, which is higher than would be predicted for patients with this level of high-risk disease, although no differences were seen in cohorts that received or did not receive IL-2. Additional data generated from this trial definitively demonstrated that in vivo administration of IL-2 results in expansion of Tregs. Zhang et al. showed that CD4+CD25+ Tregs underwent homeostatic peripheral expansion during immune reconstitution, that IL-2 therapy expanded this subset, and that this expansion was further augmented by lymphopenia3.
In a leukemia trial that included 7 children with high-risk acute myeloid leukemia or acute lymphoblastic leukemia (ALL) in cytologic remission, patients received up to 6 subcutaneous injections of a tumor vaccine consisting of leukemic blasts admixed with skin fibroblasts transduced with adenoviral vectors encoding IL-2 and CD40 ligand. Eight patients remained disease-free for a range of 27 to 62 months after treatment, with a 5-year overall survival of 90%31. Thus, IL-2 therapy is well tolerated in pediatric patients despite being heavily pretreated for their primary disease, and may enhance the cytotoxicity of both T-cell and non-T cell subsets, but Treg expansion remains an issue.
Limitations of IL-2
There remains considerable debate about the dosing, schedule, and timing of IL-2 administration in relation to vaccination. In general, high dose IL-2 appears to be associated with better clinical responses in melanoma (Table 1), although there is minimal data directly comparing IL-2 dosing. What is well documented is that higher doses of IL-2 cause appreciable toxicity, namely capillary leak2. In addition, elevation of IL-6 has been associated with IL-2-induced mental depression32 and IL-2 therapy can result in autoimmune toxicities, including vitiligo, Type I diabetes or autoimmune thyroiditis33, perhaps indicative of the potential for these cytokines to induce tumor responses against self-antigens. IL-10 production may be a direct result of Treg stimulation, and may hamper antitumor effects. In addition, IL-2 mediates activation-induced cell death, which could also hamper T-cell responses to a vaccine2. Lastly, there is some concern that IL-2 may signal tumors themselves34–42, particularly lymphoid-derived cancers and some adult carcinomas (Table 2).
Table 2.
Malignancies implicated to utilize gamma(c) cytokines
| Cytokine | Malignancy | Evidence | Ref. |
|---|---|---|---|
| IL-2 | Hodgkin disease | IL-2R+ or CD25+ by IHC | [34, 35] |
| B- and T-cell lymphomas | IL-2+ or IL-2R+ or CD25+ by IHC IL-2mRNA in CD25+ cells IL-2 can signal and promotes proliferation |
[34–37, 42] | |
| T-cell leukemias | Aberrant expression CD25mRNA | [38] | |
| B-cell CLL | CD25+ and CD122+ by antibody and mRNA analysis IL-2 induces proliferation of CD25+ cells |
[39] | |
| Head and neck carcinoma | Surface IL-2+ and CD122+ and intracellular IL-2+ by flow cytometry Anti-CD25 treatment induces G1 arrest and induces apoptosis in vitro |
[40, 41] | |
| Gastric carcinoma | Surface IL-2+ and CD122+ and intracellular IL-2+ by flow cytometry | [40] | |
| Squamous cell lung carcinoma | IL-2+ and CD25+ by IHC | [37] | |
| IL-7 | Acute B-cell leukemia | IL-7R mRNA+, IL-7R protein+ and shows in vitro kinase activity Growth inhibition by rapamycin reversed by IL-7 CD127 is alternatively spliced |
[58, 60, 61, 64] |
| Acute T-cell leukemia | Notch1 binds to IL-7R promoter, regulates IL-7R transcription and CD127 expression | ||
| Hodgkin disease | IL-7R+ by flow cytometry and IHC IL-7 stimulates growth in colony assays IL-7 prevents apoptosis in serum-free assays |
[62] | |
| Lung carcinoma | IL-7R mRNA+, IL-7R protein+ and in vitro kinase activity IL-7 induces VEGF-D and promotes lymphangiogenesis |
[58, 59] | |
| Brain tumors | IL-7R is alternatively spliced | [64] | |
| IL-15 | Large granular leukemia | IL-15 stimulates proliferation IL-15 induces all known signaling deregulations |
[105, 106] |
| CLL | IL-15 causes receptor signaling, proliferation and prevents apoptosis Cells stimulated with CD40 exhibit increased expression of IL-15R |
[107] | |
| Pediatric ALL | High IL-15 expression correlates with CNS involvement | [111] | |
| Cutaneous T cell lymphoma | IL-15 can signal and promote proliferation | [42] | |
| Renal cell carcinoma | IL-15R+ by flow cytometry and RT-PCR IL-15 can signal IL-15R |
[109] | |
| Head and neck carcinoma | IL-15Ra+ by RIA | [108] | |
| IL-21 | T cell leukemia | IL-21R+ by flow cytometry and RT-PCR IL-21 induces signaling and proliferation |
[140] |
| Hodgkin disease | IL-21+ by flow cytometry, RT-PCR and IHC IL-21R+ by flow cytometry IL-21 signals STAT5 to cause proliferation IL-21 protects cells from apoptosis |
[156, 157] | |
| B- and T-cell lymphomas | IL-21R+ by flow cytometry IL-21 causes proliferation |
[140] | |
| Multiple myeloma | IL-21 signals and is a growth factor via an IGF-1 autocrine loop | [158] |
IHC = Immunohistochemistry, CNS = central nervous system, RT-PCR = reverse transcriptase polymerase chain reaction, RIA = radioimmunoassay
In summary, most vaccines tested with IL-2 do not do better than IL-2 alone, although there are many factors that can affect outcome, including the type of vaccine, dose and schedule of IL-2, and antigen targeted. Importantly, the relative expansion of Tregs may also be hampering responses. Future work will need to address limiting expansion of this subset. In addition, almost all of the patients treated in these trials had measurable, and often bulky, tumors at the time of enrollment. It is possible that vaccines and IL-2 may work better in a minimal residual disease (MRD) setting, and future work should focus on using these two modalities as a means to minimize relapse. With regards to scheduling and timing of IL-2, in most clinical trials, IL-2 is usually given at the same time or following vaccination (Table 1). IL-2 might be more effective if given before vaccine administration, so the milieu will promote proinflammatory immune responses10, or if IL-2 is delayed until after the T cell contraction phase43. Overall, the relatively low rate of clinical responses to vaccines with IL-2, regardless of dosing or schedule, indicates that IL-2 may not be optimal as an adjuvant.
Interleukin-7
Background
IL-7 is produced by a variety of cell types and tissues, but not by lymphocytes themselves, and serum IL-7 levels are inversely correlated with lymphocyte counts. IL-7 is involved in the maintenance and survival of alpha-beta T cells, the development of B cells and gamma-delta T cells, and may play a role in the biology of DCs and monocytes44,45 (Figure 1). IL-7 does not appear to support NK cells. Thus, IL-7 plays a critical role in lymphocyte homeostasis as indicated by markedly diminished lymphocyte counts in IL-7 and IL-7 receptor gene deleted mice and the severe combined immunodeficiency associated with IL-7 receptor mutations in humans. An extensive review of IL-7 biology and signaling can be found elsewhere44,46,47, but this review will focus on potential clinical utility of recombinant human (rh) IL-7 as an agent for immunorestoration or as adjuvant therapy for vaccines or adoptively transferred T cells. Preliminary data thus far demonstrates that IL-7 therapy enhances immune reconstitution, but without stimulating Treg expansion or inducing capillary leak, as occurs with IL-2.
Clinical Trials with IL-7
The first rhIL-7 phase I trial reported 12 patients with metastatic cancers. The four tested doses were 3, 10, 30, and 60 μg/kg given subcutaneously every 3 days for a total of eight doses. Patients also received the melanoma antigen peptide vaccines gp100 and MART-1 in incomplete Freund’s adjuvant subcutaneously. The therapy was well tolerated and no MTD was reached. While no anti-tumor effects were observed, rhIL-7 was given for limited time combined with a limited number of vaccines, therefore limiting the conclusions that could be drawn regarding its capacity to enhance vaccine responses. Of note, CD4+ and CD8+ T-cell subsets increased in this trial in a dose-dependent manner. However, there was a relative decrease in Tregs, making this cytokine distinct from IL-2. There was also an increase in B cell precursors in the bone marrow of some patients, but no changes in B cell numbers were noted peripherally48.
The second rhIL-7 phase I trial reported 16 patients with refractory malignancies using the same doses as the first trial, but IL-7 was given every other day for 14 days and no vaccines were administered. The therapy was well tolerated and no MTD was reached. No anti-tumor effects were observed, however rhIL-7 increased both CD4+ and CD8+ T cells, including central memory subsets, in a dose-dependent fashion, and these increases lasted for weeks after discontinuation of the cytokine. The mechanism of expansion appeared to be augmentation of peripheral cycling with a propensity for cycling of naïve populations. While enhanced thymic output could not be definitively discerned, rhIL7 induced increased T cell repertoire diversity as measured by spectratyping, presumably due to enhanced cycling of recent thymic emigrants. Notably, Tregs were not increased, making this clearly cytokine distinct from IL-249.
Lastly, a phase I trial using melanoma cells engineered to express IL-7 lead to an increase in melanoma-reactive T cells in three out of six patients. Minor antitumor responses were observed in two patients50. While this trial is not the equivalent of giving IL-7 directly, it further demonstrated that IL-7 is well tolerated in vivo, and is effective in mediating effector T cell expansion without associated Tregs.
Potential Antitumor Applications of IL-7
While IL-7 alone does not seem to eliminate tumors directly, rationale combination with other immunotherapies may be beneficial. In preclinical models, IL-7 therapy potently enhances vaccine-mediated immunity51,52. Combining intralesional IL-7 with other therapies, such as radiofrequency ablation (RFA), induces immune responses to breast tumors, inhibits tumor development and lung metastasis, and reduces myeloid-derived suppressor cells53. Combining IL-7 with local hyperthermia also enhances anti-tumor activity in mice with melanoma54 and combining IL-7 and lymphocytes results in prolonged survival from colon cancer55. In a preclinical neuroblastoma xenograft model, combining IL-7 and gamma-delta T cells with an anti-GD2 antibody significantly improved survival56. Thus in both adult and pediatric solid tumor models, IL-7 has the capability to be an effective adjuvant. Recently, adjuvant IL-7 was shown to improve vaccine mediated survival in a spontaneously occurring murine tumor model via enhanced Th17 differentiation and reduced T cell-intrinsic inhibitory networks51. Finally, IL-7 may have utility after allogeneic HSCT, where it may enhance graft-versus-leukemia (GVL) effects by potentiating alloreactive T cells57. Thus, the available preclinical data and limited data from clinical trials would indicate that via multiple mechanisms, IL-7 is a very promising agent to enhance overall immune competence and, potentially, tumor specific immune responses. The absence of Treg expansion and the lack of toxicity observed in this clinic would suggest that IL-7 offers definite advantages over IL-2 as an adjuvant.
Potential Limitations of IL-7 Therapy
A potential concern regarding IL-7 therapy is that it may signal tumors directly58–62, promoting growth/survival (Table 2). CD127 expression has been reported on some adult solid tumors58, but not on pediatric solid tumors, but it is not clear if these tumors have the capacity to signal through IL-7. IL-7 does play a role in either the initiation or maintenance of some leukemias and lymphomas and therefore will need to be used with extreme caution in immunotherapy regimens involving lymphoid malignancies. It could be that malignant tissues alternatively splice IL-7, as shown in neuronal tumors and pediatric ALL63,64, which suggests that some tumors could generate their own supply of IL-7 for survival or possibly use an isoform as a means of local IL-7 receptor blockade on effector cells. Some neuronal tumors also alternatively splice the IL-7 receptor, suggesting the tumor could modulate their ability to respond to exogenous IL-764.
While IL-7 has been shown to enhance GVL responses after allogeneic HSCT, it may also exacerbate Graft-Versus-host-Disease (GVHD)65,66. Thus, the use of IL-7 in the allogeneic setting may be most effective in the setting of T cell-depleted grafts67. IL-7 overexpression has been described to create an osteoclastogenic microenvironment within the bone marrow, which promotes the commitment of precursors towards the osteoclast lineage, leading to bone loss68. Lastly, additional pre-clinical work has shown that IL-7 therapy may generate a suppressive DC that does not present antigen effectively45. Careful selection of tumors along with close monitoring of bone density and the development of autoimmunity may be necessary in future trials. However, the overall experience with IL-7 thus far would indicate that clinical trials with this cytokine in multiple settings including as a vaccine adjuvant are warranted.
Interleukin-15
Background
IL-15 is constitutively expressed by a variety of cell types and tissues, but in contrast to IL-2, is mainly membrane bound. A thorough review of IL-15 biology and receptor physiology has been described elsewhere1,2,69. IL-15 and IL-2 exhibit similar immune effects and share the IL-2 receptor subunits IL-2Rbeta and IL-2Rgamma(c), but each cytokine has a separate alpha receptor (Ra). One unique feature of IL-15 is the requirement for cross presentation by IL-15Ra in order to induce optimal biologic activity. Unlike most cytokines that function as soluble mediators, IL-15 appears to function primarily as a “cell associated” molecule and therefore is highly dependent upon an available reservoir of IL15Ra+ cells for optimal biologic activity. It is possible that this feature of IL-15 biology will have important implications for how best to utilize this cytokine as a therapeutic agent70.
IL-15 is required for the differentiation of NK cells71, and plays a role in maintaining and expanding CD8+ T cells (particularly memory subsets)72–74, NK cells75, NKT cells76,77, interferon-killer DCs78,79 and gamma-delta T cells80,81. In addition to effects on T cells and NK cells, IL-15 may also sustain B cells82 and convert polymorphonuclear cells into APCs83 (Figure 1). Finally, there has been a number of reports exploring NK cell-DC cross talk, and IL-15 is presented by DCs to NK cells to enhance survival84,85. Based upon these properties, investigators have incorporated IL-15 with or onto artificial APCs to expand NK cells86–88. IL-15 has not been tested in clinical trials yet, but is being developed by the NCI for clinical use2. Thus, exploration of preclinical data is warranted to gain a full understanding of the potential impact in the clinic.
IL-15 and Preclinical Tumor Models
While IL-15 may have direct anti-tumor effects, most studies demonstrate that it acts as an adjuvant to enhance anti-tumor immunity, as was described with IL-2 and IL-7. IL-15 is superior to IL-2 in lung adenocarcinoma models89, and can improve immunity against colon cancer90,91. Moreover, IL-15 treatment increased amphoterin (HMGB1) secretion by colon cancer cells, which was associated with a depletion of tumor-associated macrophages92. In melanoma, IL-15 can mediate regression of established tumor93, and may also enhance tumor-resident CD8+ T cells rather than attract newly infiltrated T cells94. When combined with IL-7 or IL-12, it may even be better than the “gold standard” of IL-2 to enhance T cell-mediated killing of melanoma95,96. NK cells show enhanced killing of Ewing sarcoma cells after IL-15 administration97. RFA of breast tumors combined with intralesional IL-15 (and IL-7) inhibits tumor development and metastasis53. IL-15 enhances NK cell cytotoxicity of human glioblastoma cells, which are resistant to freshly isolated NK cells98. IL-15 also can reverse the unresponsiveness to the antigen WT-1 in prostate cancer lines, leading to restored expansion and gamma interferon production of WT1-specific T cells99. Thus IL-15 may enhance anti-tumor immune responses to a wide variety of pediatric and adult malignancies.
There have been numerous preclinical studies exploring IL-15 as a vaccine adjuvant. Although the majority has been in infection models, a number of reports of the adjuvant effect of IL-15 have been published. One important observation is that IL-15 can revert tolerant T cells to become effectors100. Adjuvant use of IL-15 can enhance vaccine responses to both dominant and subdominant tumor antigens101. Recently, IL-15 administered after a gene-modified vaccine resulted in enhanced anti-tumor activity in a murine melanoma model96. After allogeneic HSCT, IL-15 seems to upregulate NK cell activating receptors102, and administration of IL-15 with IL-2 enhanced NK-DLI-mediated GVL103. Importantly, although IL-15 is thought to act primarily on mature T cells, it may prove to be beneficial after T cell-depleted HSCT104. Based on the available pre-clinical data, IL-15 would appear to be well suited as an adjuvant to cancer vaccines.
Potential Limitations of IL-15 Therapy
The main limitation is that IL-15 is not yet readily available. Despite the fact IL-15 was cloned in 1994, rhIL-15 remains under development by the NCI2. Some caution should be expressed with IL-15 administration in certain tumor types42,105–108, since there is both evidence of IL-15R expression and involvement in tumoral progression (Table 2). Normal kidney expresses a functional IL-15 receptor109, and human RCC expresses an IL-15R that seems to be directly involved in renal tumoral progression110. In pediatric ALL, high IL-15 expression correlates with CNS involvement111, but those children with high IL-15Ra expression have a significantly better probability of survival at 5 years112. Mice that have transgenic overexpression of IL-15 also develop a fatal large granular leukemia113.
Besides directly stimulating tumor growth, IL-15 may also enhance endogenous immunosuppressive pathways. Umbilical cord blood-derived Tregs stimulated with IL-2 and IL-15 express higher levels of cytotoxic T lymphocyte antigen-4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor superfamily member number 18 (GITR), membrane bound transforming growth factor (TGF)-beta and FOXp3, leading to higher production of IL-10 and TGF-beta114. Even Tregs from peripheral blood can be generated and sustained partially with IL-15 in the absence of IL-2115,116. After allogeneic HSCT, trans-presentation of donor-derived IL-15 is needed for acute GVHD but not for GVL effects117. Given the described effects of IL-15 in sustaining memory T cells, there is concern that IL-15 could potentiate GVHD by supporting alloreactive memory T cells118, and IL-15 has been shown to exacerbate xenogeneic GVHD119. Lastly there is an extensive literature on the effects of IL-15 on total body fat mass as well as promoting autoimmunity69. As is the case with IL-7, there is great promise for enhancing anti-tumor immunity with IL-15, but the potential to signal tumors, and possibly Tregs, as well as to the potential for induction of autoimmunity, remain valid concerns.
Interleukin-21
Background
IL-21 is homologous to IL-15, but the receptor for IL-21 is comprised of a unique subunit designated IL-21Ra and the IL-2Rgamma(c)120,121 and there is no evidence that IL-21 requires trans-presentation for biologic activity. IL-21Ra is expressed on most mature lymphocyte populations (Figure 1). Production of IL-21 is restricted to activated CD4+ T helper cells122,123. IL-21 appears to play important roles in modulating responses of lymphocytes to other cytokines. While IL-21 alone does not affect receptor expression, IL-21 can synergize with IL-2 to up-regulate several surface receptors, including NKG2A, CD25, CD86 and CD69124. In certain tumor models IL-21-enhanced tumor rejection is NKG2D dependent125, however IL-21 does not support NK cells, and in fact, has been shown to limit NK cell expansion and induce apoptosis126,127. IL-21 alone does not induce T cell proliferation, however IL-21 can enhance the effects of other stimuli of proliferation, such as other gamma(c) cytokines128–130. IL-21 also has a role in B cell proliferation128 but may uniquely also induce B cell apoptosis131. IL-21 has also been shown to induce IL-10 production in models of lupus, suggesting that like IL-2, it can also contribute to immunosuppressive activity132. Thus, the available pre-clinical data would suggest that IL-21 may work best in combination with other gamma(c) cytokines in the adjuvant setting.
Clinical Trials with IL-21
There have been 3 clinical trials with IL-21. In a phase I trial of 43 patients with metastatic melanoma and RCC, IL-21 was administered in two 5-day cycles on days 1 through 5, and 15 through 19, of a treatment course. Doses ranged from 3 to 100 ug/kg/dose, and an expanded cohort was treated at the MTD, estimated to be 30 ug/kg. Twenty-eight patients were treated in the expanded cohort. Twelve patients received up to five additional two-cycle courses of treatment without cumulative toxicity, except for one patient with reversible grade IV hepatotoxicity. Antitumor activity was observed in both melanoma (1CR, 4%) and RCC (4PR, 21%)133.
In another open-label, two-arm, dose escalation phase I trial of IL-21 involving 29 patients with metastatic melanoma, dose levels from 1 to 100 ug/kg were utilized in two parallel treatment regimens: thrice weekly for 6 weeks (3/wk) or three cycles of daily dosing for 5 days followed by 9 days of rest (5+9). The MTD was also 30 ug/kg for both regimens. One PR was observed after the 3/wk regimen, and became a CR 3 months later134.
In a phase II, open-label, single-arm, two-stage trial study of IL-21 (30 ug/kg/dose) was administered in 8-week cycles (5+9) in patients with metastatic melanoma. No toxicity was observed, and the best tumor response included 1 CR and 1 PR, both with lung metastases135. Pharmacodynamic studies show that IL-21 affects the serum levels of several cytokines, chemokines, acute-phase proteins and cell adhesion proteins in a dose-dependent fashion136. In the (5+9) regimen, IL-21 induced a dose-dependent decrease in circulating NK cells and T cells, followed by a return to baseline in resting periods. In both CD8+ T cells and NK cells, up-regulation of perforin and granzyme B mRNA was observed. Finally, cytotoxicity assays showed that IL-21 enhanced the ability of NK cells to kill sensitive targets ex vivo137.
Potential Antitumor Applications of IL-21
IL-21 may have direct anti-tumor effects. For example, the majority of chronic lymphocytic leukemia (CLL) patients have surface IL-21Ra, its expression correlates with apoptosis107 and IL-21 counteracts the proliferative and antiapoptotic signals delivered by IL-15 to CLL B cells138. In addition to its pro-apoptotic effect, IL-21 promotes NK cell-mediated antibody-dependent cellular cytotoxicity against rituximab-coated CLL cells in vitro139. While follicular lymphoma cells show high levels of IL-21R, addition of the cytokine inhibits proliferation and induced apoptosis140. Gene-modified melanoma cells that express IL-21 grow slower than nonmodified cells in vitro and in vivo141. IL-21 has also been shown to exert activities on vascular endothelial cells (ECs), leading to decreased angiogenesis related gene expression142, decreased proliferation and sprouting of activated ECs after IL-21 treatment, disturbing vessel architecture and negatively affecting vessel outgrowth. A murine myeloma cell vaccine containing IL-21 plasmid DNA induced significant tumor regression and prolonged survival143. IL-21-secreting RENCA cells were efficiently rejected following subcutaneous injection into syngeneic mice144. Similar results were seen in a mouse bladder carcinoma genetically modified to express IL-21145. Finally, using a glioblastoma transduced to express IL-21, 100% of the animals rejected the tumor, and 76% of these animals survived a subsequent tumor rechallenge, while other transduced cytokine genes were not as effective146.
IL-21 may also improve the potency of effector cells and other gamma(c) cytokines. For instance, administering IL-21 locally to melanoma tumors enhanced the therapeutic effects of adoptively transferred gp100-specific T cells, and was synergistic with IL-2, leading to an increased proliferation of local CD8+ T cells and decreased accumulation of Tregs within the tumor microenvironment147. IL-21 also improves expansion and effector function of gamma-delta T cells, and reverses expression of inhibitory receptors. IL-21 can be combined with IL-2 to enhance gamma-delta T cell-mediated antitumor responses148. Use of IL-21 and IL-2 in culture up-regulate cytokine production of activated tumor-draining lymph node cells, and enhances their therapeutic efficacy against established pulmonary metastatic fibrosarcomas. Animals treated with combined IL-21 and IL-2 showed protective immunity against tumor rechallenge, with expansion of memory T cells, antibody production, and significantly elevated serum levels of IFN-gamma and IL-10149.
Besides enhancing IL-2 therapy, IL-21 may also improve the effectiveness of other cytokines and immunotherapies. Combining alpha interferon and IL-21 increases NK cell and CD8+ T-cell-mediated cytotoxicity in an experimental model of RCC, leading to inhibition of tumor growth and an increased survival150. IL-21 can also significantly augment IL-7-induced expansion of cytotoxic T cells, possibly by preventing the cytokine-induced down-regulation of CD127 on antigen-stimulated T cells, results which suggest that IL-21 may also play a cooperative role with IL-7 in modulating primary CD8+ T-cell responses151. Several monoclonal antibodies targeting TAAs also have improved antitumor activities in mice when used in combination with IL-21152 and human NK cells cultured with IL-21 and human breast cancer cells coated with trastuzumab showed enhanced lytic activity153. Lastly, in regards to a pediatric tumor, vaccinating with IL-21-gene-modified cells in a syngeneic metastatic neuroblastoma model demonstrated a reduction of microvessels in late metastases from therapeutically vaccinated mice. A role of survivin as a tumor antigen was suggested since a specific T cell response against this antigen was induced154.
Interestingly, the route of IL-21 administration may be critical. Whereas both subcutaneous (SC) and intraperitoneal (IP) routes of IL-21 administration significantly inhibit growth of small, established RCC and melanoma tumors, only SC therapy significantly inhibited the growth of large, established tumors. Greater bioavailability and significant drainage of IL-21 to regional lymph nodes was observed following SC administration, which could account for the apparent increase in anti-tumor activity. In the RCC model, SC administration of IL-21 led to a significantly higher density of tumor infiltrating CD8+ T cells compared to IP155.
Limitations to IL-21 Therapy
As with the other gamma(c) cytokines, IL-21 receptor has been observed on multiple tumor types156–158 and IL-21 has contributed to tumoriogenesis (Table 2). IL-21 shows divergent effects depending on the cell origin: growth stimulation in B cell lymphoma cell lines and adult T cell leukemia/lymphoma cell lines but induction of apoptosis in follicular lymphoma140. IL-21 has also been implicated in the pathogenesis of autoimmunity in a number of models159. As with the other gamma(c) cytokines, care in selecting the relevant tumor types as well as care in not to enhance Treg activity or cause autoimmunity is warranted.
Conclusion
Although initial clinical trials using IL-2 as a vaccine adjuvant demonstrated only modest effects in the clinic, combination immunotherapies using newer gamma(c) cytokines to promote NK cell and T-cell expansion and effector function are promising strategies to enhance immunotherapy of tumors. A number of different vaccine strategies in both preclinical and clinical studies have shown potentiation with concomitant IL-7, IL-15 and IL-21. These results have involved various regimens of adjuvant cytokine therapy, with differences in dosing, time of administration and schedule leading to different outcomes. Further research is needed to determine the most potent vehicles of vaccination as well as effective doses and schedules for cytokine delivery. Combinations of cytokines may be warranted. Future T cell-based immunotherapies will likely combine regimens that optimize vaccination and/or adoptive cell therapy with growth-promoting cells that can augment anti-tumor immunity while limiting autoimmunity responses. Caution needs to be exercised that tumors themselves are not signaling by these cytokines so that relapse is not promoted, and patients should be monitored for autoimmunity where possible.
Acknowledgments
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
This work was supported by intramural research funds at the National Cancer Institute (C.L.M.).
Footnotes
Publisher's Disclaimer: The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Literature Cited
- 1.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine & Growth Factor Reviews. 2002;13:169–183. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 2.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nature Reviews of Immunology. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PB. Combining a peptide vaccine with high-dose interleukin-2. J Clin Oncol. 2008;26:2250–2251. doi: 10.1200/JCO.2007.15.7826. [DOI] [PubMed] [Google Scholar]
- 5.Coppin C. Immunotherapy for renal cell cancer in the era of targeted therapy. Expert Review of Anticancer Therapy. 2008;8:907–919. doi: 10.1586/14737140.8.6.907. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nature medicine. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Impact of Cytokine Administration on the Generation of Antitumor Reactivity in Patients with Metastatic Melanoma Receiving a Peptide Vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JD, Niedzwiecki D, Carson WE, et al. Phase 2 study of the g209-2M melanoma peptide vaccine and low-dose interleukin-2 in advanced melanoma. Journal of Immunotherapy. 2006;29:95–101. doi: 10.1097/01.cji.0000195295.74104.ad. [DOI] [PubMed] [Google Scholar]
- 9.Sosman JA, Carrillo C, Urba WJ, et al. Three Phase II Cytokine Working Group Trials of gp100 (210M) Peptide Plus High-Dose Interleukin-2 in Patients With HLA-A2-Positive Advanced Melanoma. J Clin Oncol. 2008;26:2292–2298. doi: 10.1200/JCO.2007.13.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Immunologic and Clinical Outcomes of Vaccination With a Multiepitope Melanoma Peptide Vaccine Plus Low-Dose Interleukin-2 Administered Either Concurrently or on a Delayed Schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 11.Hersey P, Halliday G, Farrelly M, DeSilva C, Lett M, Menzies S. Phase I/II study of treatment with matured dendritic cells with or without low dose IL-2 in patients with disseminated melanoma. Cancer Immunology, Immunotherapy. 2008;57:1039–1051. doi: 10.1007/s00262-007-0435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panelli MC, Wunderlich J, Jeffries J, et al. Phase 1 study in patients with metastatic melanoma of immunization with dendritic cell presenting epitopes derived from the melanoma-associated antigens MART-1 and gp100. Journal of Immunotherapy. 2000;23:487–498. doi: 10.1097/00002371-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Redman BG, Chang AE, Whitfield J, et al. Phase Ib trial assessing autologous, tumor-pulsed dendritic cells as a vaccine administered with or without IL-2 in patients with metastatic melanoma. Journal of Immunotherapy. 2008;31:591–598. doi: 10.1097/CJI.0b013e31817fd90b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagayama H, Sato K, Morishita M, et al. Results of a Phase I Clinical Study Using Autologous Tumour Lysate-pulsed Monocyte-derived Mature Dendritic Cell Vaccinations for Stage IV Malignant Melanoma Patients Combined with Low Dose interleukin-2. Melanoma Research. 2003;13:521–530. doi: 10.1097/00008390-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 15.Haenssle H, Krause SW, Emmert S, et al. Hybrid cell vaccination in metastatic melanoma: clinical and immunologic results of a phase I/II study. Journal of Immunotherapy. 2004;27:147–155. doi: 10.1097/00002371-200403000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Escobar A, Lopez M, Serrano A, et al. Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients. Clinical & Experimental Immunology. 2005;142:555–568. doi: 10.1111/j.1365-2249.2005.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei Y, Sticca RP, Holmes LM, et al. Dendritoma vaccination combined with low dose interleukin-2 in metastatic melanoma patients induced immunological and clinical responses. International Journal of Oncology. 2006;28:585–593. [PubMed] [Google Scholar]
- 18.Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive Transfer of Vaccine-Induced Peripheral Blood Mononuclear Cells to Patients with Metastatic Melanoma following Lymphodepletion. J Immunol. 2006;177:6527–6539. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lotem M, Shiloni E, Pappo I, et al. Interleukin-2 improves tumour response to DNP-modified autologous vaccine for the treatment of metastatic malignant melanoma. British Journal of Cancer. 2004;90:773–780. doi: 10.1038/sj.bjc.6601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg S, Zhai Y, Yang J, et al. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Recombinant Fowlpox Viruses Encoding the Anchor-modified gp100 Melanoma Antigen Can Generate Antitumor Immune Responses in Patients with Metastatic Melanoma. Clin Cancer Res. 2003;9:2973–2980. [PMC free article] [PubMed] [Google Scholar]
- 22.Dudek AZ, Mescher MF, Okazaki I, et al. Autologous large multivalent immunogen vaccine in patients with metastatic melanoma and renal cell carcinoma. American journal of clinical oncology: cancer clinical trials. 2008;31:173–181. doi: 10.1097/COC.0b013e3181573e6b. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson S, Guile K, John J, et al. A randomized phase II trial of SRL172 (Mycobacterium vaccae) +/− low-dose interleukin-2 in the treatment of metastatic malignant melanoma. Melanoma Research. 2003;13:389–393. doi: 10.1097/00008390-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Smith FO, Downey SG, Klapper JA, et al. Treatment of Metastatic Melanoma Using Interleukin-2 Alone or in Conjunction with Vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousseau RF, Haight AE, Hirschmann-Jax C, et al. Local and systemic effects of an allogeneic tumor cell vaccine combining transgenic human lymphotactin with interleukin-2 in patients with advanced or refractory neuroblastoma. Blood. 2003;101:1718–1726. doi: 10.1182/blood-2002-08-2493. [DOI] [PubMed] [Google Scholar]
- 26.Russell HV, Strother D, Mei Z, et al. Phase I trial of vaccination with autologous neuroblastoma tumor cells genetically modified to secrete IL-2 and lymphotactin. Journal of Immunotherapy. 2007;30:227–233. doi: 10.1097/01.cji.0000211335.14385.57. [DOI] [PubMed] [Google Scholar]
- 27.Russell HV, Strother D, Mei Z, et al. A phase 1/2 study of autologous neuroblastoma tumor cells genetically modified to secrete IL-2 in patients with high-risk neuroblastoma. Journal of Immunotherapy. 2008;31:812–819. doi: 10.1097/CJI.0b013e3181869893. [DOI] [PubMed] [Google Scholar]
- 28.Yu A, Gilman A, Ozkaynak M, et al. A phase III randomized trial of the chimeric anti-GD2 antibody ch14.18 with GM-CSF and IL2 as immunotherapy following dose intensive chemotherapy for high-risk neuroblastoma: Children’s Oncology Group (COG) study ANBL0032. J Clin Oncol. 2009:27. abstr 10067z. [Google Scholar]
- 29.Dagher R, Long L, Read E, et al. Pilot trial of tumor-specific peptide vaccination and continuous infusion interleukin-2 in patients with recurrent Ewing sarcoma and alveolar rhabdomyosarcoma: An inter-institute NIH study. Medical and Pediatric Oncology. 2002;38:158–164. doi: 10.1002/mpo.1303. [DOI] [PubMed] [Google Scholar]
- 30.Mackall CL, Rhee EH, Read EJ, et al. A Pilot Study of Consolidative Immunotherapy in Patients with High-Risk Pediatric Sarcomas. Clin Cancer Res. 2008;14:4850–4858. doi: 10.1158/1078-0432.CCR-07-4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rousseau RF, Biagi E, Dutour A, et al. Immunotherapy of high-risk acute leukemia with a recipient (autologous) vaccine expressing transgenic human CD40L and IL-2 after chemotherapy and allogeneic stem cell transplantation. Blood. 2006;107:1332–1341. doi: 10.1182/blood-2005-03-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell MS. Immunotherapy as part of combinations for the treatment of cancer. International Immunopharmacology Combination immunotherapy. 2003;3:1051–1059. doi: 10.1016/S1567-5769(03)00019-5. [DOI] [PubMed] [Google Scholar]
- 33.Chianese-Bullock KA, Woodson EM, Tao H, et al. Autoimmune Toxicities Associated with the Administration of Antitumor Vaccines and Low-Dose Interleukin-2. Journal of Immunotherapy. 2005;28:412–419. doi: 10.1097/01.cji.0000171314.00924.2b. [DOI] [PubMed] [Google Scholar]
- 34.Strauchen JA, Breakstone BA. IL-2 receptor expression in human lymphoid lesions. Immunohistochemical study of 166 cases. The American journal of pathology. 1987;126:506–512. [PMC free article] [PubMed] [Google Scholar]
- 35.Sheibani K, Winberg CD, van de Velde S, Blayney DW, Rappaport H. Distribution of lymphocytes with interleukin-2 receptors (TAC antigens) in reactive lymphoproliferative processes, Hodgkin’s disease, and non-Hodgkin’s lymphomas. An immunohistologic study of 300 cases. The American journal of pathology. 1987;127:27–37. [PMC free article] [PubMed] [Google Scholar]
- 36.Peuchmaur M, Emilie D, Crevon MC, et al. IL-2 mRNA expression in Tac-positive malignant lymphomas. The American journal of pathology. 1990;136:383–390. [PMC free article] [PubMed] [Google Scholar]
- 37.Olejniczak K, Kasprzak A. Medical science monitor. Vol. 14. 2008. Biological properties of interleukin 2 and its role in pathogenesis of selected diseases--a review; pp. RA179–189. [PubMed] [Google Scholar]
- 38.Horiuchi S, Koyanagi Y, Tanaka Y, et al. Altered interleukin-2 receptor alpha-chain is expressed in human T-cell leukaemia virus type-I-infected T-cell lines and human peripheral blood mononuclear cells of adult T-cell leukaemia patients through an alternative splicing mechanism. Immunology. 1997;91:28–34. doi: 10.1046/j.1365-2567.1997.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsilivakos V, Tsapis A, Kakolyris S, Iliakis P, Perraki M, Georgoulias V. Characterization of interleukin 2 receptors on B-cell chronic lymphocytic leukemia cells. Leukemia. 1994;8:1571–1578. [PubMed] [Google Scholar]
- 40.Lin WC, Yasumura S, Suminami Y, et al. Constitutive production of IL-2 by human carcinoma cells, expression of IL-2 receptor, and tumor cell growth. The journal of immunology. 1995;155:4805–4816. [PubMed] [Google Scholar]
- 41.Kuhn DJ, Dou QP. Direct inhibition of interleukin-2 receptor alpha-mediated signaling pathway induces G1 arrest and apoptosis in human head-and-neck cancer cells. Journal of cellular biochemistry. 2005;95:379–390. doi: 10.1002/jcb.20446. [DOI] [PubMed] [Google Scholar]
- 42.Marzec M, Halasa K, Kasprzycka M, et al. Differential Effects of Interleukin-2 and Interleukin-15 versus Interleukin-21 on CD4+ Cutaneous T-Cell Lymphoma Cells. Cancer Res. 2008;68:1083–1091. doi: 10.1158/0008-5472.CAN-07-2403. [DOI] [PubMed] [Google Scholar]
- 43.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 44.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99:3892–3904. doi: 10.1182/blood.v99.11.3892. [DOI] [PubMed] [Google Scholar]
- 45.Guimond M, Veenstra RG, Grindler DJ, et al. Interleukin 7 signaling in dendritic cells regulates the homeostatic proliferation and niche size of CD4+ T cells. Nat Immunol. 2009;10:149–157. doi: 10.1038/ni.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palmer MJ, Mahajan VS, Trajman LC, Irvine DJ, Lauffenburger DA, Chen J. Interleukin-7 receptor signaling network: an integrated systems perspective. Cellular & molecular immunology. 2008;5:79–89. doi: 10.1038/cmi.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Capitini CM, Chisti AA, Mackall CL. Modulating T-cell homeostasis with IL-7: preclinical and clinical studies. Journal of Internal Medicine. 2009;266:141–153. doi: 10.1111/j.1365-2796.2009.02085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenberg SA, Sportès C, Ahmadzadeh M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. Journal of Immunotherapy. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sportes C, Hakim FT, Memon SA, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moller P, Sun Y, Dorbic T, et al. Vaccination with IL-7 gene-modified autologous melanoma cells can enhance the anti-melanoma lytic activity in peripheral blood of patients with a good clinical performance status: a clinical phase I study. The British journal of cancer. 1998;77:1907–1916. doi: 10.1038/bjc.1998.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pellegrini M, Calzascia T, Elford AR, et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med. 2009 doi: 10.1038/nm.1953. advanced online publication. [DOI] [PubMed] [Google Scholar]
- 52.Colombetti S, Levy F, Chapatte L. IL-7 adjuvant treatment enhances long-term tumor antigen-specific CD8+ T cell responses following immunization with recombinant lentivectors. Blood. 2009 doi: 10.1182/blood-2008-05-155309. [DOI] [PubMed] [Google Scholar]
- 53.Habibi M, Kmieciak M, Graham L, Morales JK, Bear HD, Manjili MH. Radiofrequency thermal ablation of breast tumors combined with intralesional administration of IL-7 and IL-15 augments anti-tumor immune responses and inhibits tumor development and metastasis. Breast cancer research and treatment. 2009;114:423–431. doi: 10.1007/s10549-008-0024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu B, Shen RN, Wang WX, Broxmeyer HE, Lu L. Antitumor effect of interleukin 7 in combination with local hyperthermia in mice bearing B16a melanoma cells. Stem cells. 1993;11:412–421. doi: 10.1002/stem.5530110508. [DOI] [PubMed] [Google Scholar]
- 55.Murphy WJ, Back TC, Conlon KC, et al. Antitumor effects of interleukin-7 and adoptive immunotherapy on human colon carcinoma xenografts. The journal of clinical investigation. 1993;92:1918–1924. doi: 10.1172/JCI116785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Otto M, Barfield RC, Martin WJ, et al. Combination Immunotherapy with Clinical-Scale Enriched Human {gamma}{delta} T cells, hu14.18 Antibody, and the Immunocytokine Fc-IL7 in Disseminated Neuroblastoma. Clin Cancer Res. 2005;11:8486–8491. doi: 10.1158/1078-0432.CCR-05-1184. [DOI] [PubMed] [Google Scholar]
- 57.Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood. 2001;97:1491–1497. doi: 10.1182/blood.v97.5.1491. [DOI] [PubMed] [Google Scholar]
- 58.Cosenza L, Gorgun G, Urbano A, Foss F. Interleukin-7 receptor expression and activation in nonhaematopoietic neoplastic cell lines. Cellular Signalling. 2002;14:317–325. doi: 10.1016/s0898-6568(01)00245-5. [DOI] [PubMed] [Google Scholar]
- 59.Ming J, Zhang Q, Qiu X, Wang E. Interleukin 7/interleukin 7 receptor induce c-Fos/c-Jun-dependent vascular endothelial growth factor-D up-regulation: a mechanism of lymphangiogenesis in lung cancer. Eur J Cancer. 2009;45:866–873. doi: 10.1016/j.ejca.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Brown VI, Fang J, Alcorn K, et al. Rapamycin is active against B-precursor leukemia in vitro and in vivo, an effect that is modulated by IL-7-mediated signaling. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15113–15118. doi: 10.1073/pnas.2436348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.González-García S, García-Peydró M, Martín-Gayo E et al. CSL-MAML-dependent Notch1 signaling controls T lineage-specific IL-7R{alpha} gene expression in early human thymopoiesis and leukemia. J Exp Med. 2009 doi: 10.1084/jem.20081922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cattaruzza L, Gloghini A, Olivo K, et al. International Journal of Cancer. Vol. 9999. NA: 2009. Functional coexpression of Interleukin (IL)-7 and its receptor (IL-7R) on Hodgkin and Reed-Sternberg cells: Involvement of IL-7 in tumor cell growth and microenvironmental interactions of Hodgkin’s lymphoma. [DOI] [PubMed] [Google Scholar]
- 63.Korte A, Möricke A, Beyermann B, et al. Extensive alternative splicing of interleukin-7 in malignant hematopoietic cells: implication of distinct isoforms in modulating IL-7 activity. Journal of interferon & cytokine research. 1999;19:495–503. doi: 10.1089/107999099313947. [DOI] [PubMed] [Google Scholar]
- 64.Vudattu NK, Magalhaes I, Hoehn H, Pan D, Maeurer MJ. Expression analysis and functional activity of interleukin-7 splice variants. Genes Immun. 2008;10:132–140. doi: 10.1038/gene.2008.90. [DOI] [PubMed] [Google Scholar]
- 65.Dean RM, Fry T, Mackall C, et al. Association of Serum Interleukin-7 Levels With the Development of Acute Graft-Versus-Host Disease. J Clin Oncol. 2008;26:5735–5741. doi: 10.1200/JCO.2008.17.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sinha ML, Fry TJ, Fowler DH, Miller G, Mackall CL. Interleukin 7 worsens graft-versus-host disease. Blood. 2002;100:2642–2649. doi: 10.1182/blood-2002-04-1082. [DOI] [PubMed] [Google Scholar]
- 67.Snyder KM, Mackall CL, Fry TJ. IL-7 in allogeneic transplant: Clinical promise and potential pitfalls. Leukemia and Lymphoma. 2006;47:1222 – 1228. doi: 10.1080/10428190600555876. [DOI] [PubMed] [Google Scholar]
- 68.Roato I, Brunetti G, Gorassini E, et al. IL-7 Up-Regulates TNF-[alpha]-Dependent Osteoclastogenesis in Patients Affected by Solid Tumor. PLoS ONE. 2006;1:e124. [Google Scholar]
- 69.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: A guided tour through an expanding universe. Cytokine & Growth Factor Reviews. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 70.Oh S, Perera LP, Burke DS, Waldmann TA, Berzofsky JA. IL-15/IL-15Ralpha-mediated avidity maturation of memory CD8+ T cells. Proc Natl Acad Sci U S A. 2004;101:15154–15159. doi: 10.1073/pnas.0406649101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huntington ND, Legrand N, Alves NL, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stonier SW, Ma LJ, Castillo EF, Schluns KS. Dendritic cells drive memory CD8 T-cell homeostasis via IL-15 transpresentation. Blood. 2008;112:4546–4554. doi: 10.1182/blood-2008-05-156307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daudt L, Maccario R, Locatelli F, et al. Interleukin-15 favors the expansion of central memory CD8+ T cells in ex vivo generated, antileukemia human cytotoxic T lymphocyte lines. Journal of Immunotherapy. 2008;31:385–393. doi: 10.1097/CJI.0b013e31816b1092. [DOI] [PubMed] [Google Scholar]
- 74.Kokaji AI, Hockley DL, Kane KP. IL-15 Transpresentation Augments CD8+ T Cell Activation and Is Required for Optimal Recall Responses by Central Memory CD8+ T Cells. J Immunol. 2008;180:4391–4401. doi: 10.4049/jimmunol.180.7.4391. [DOI] [PubMed] [Google Scholar]
- 75.Huntington ND, Puthalakath H, Gunn P, et al. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ozdemir O, Savasan S. Combinational IL-2/IL-15 induction does not further enhance IL-15-induced lymphokine-activated killer cell cytotoxicity against human leukemia/lymphoma cells. Clinical Immunology. 2005;115:240–249. doi: 10.1016/j.clim.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 77.Vallabhapurapu S, Pulwolny-Budnicka S, Riemann M, et al. Rel/NF-kappaB family member RelA regulates NK1.1- to NK1.1+ transition as well as IL-15-induced expansion of NKT cells. European Journal of Immunology. 2008;38:3508–3519. doi: 10.1002/eji.200737830. [DOI] [PubMed] [Google Scholar]
- 78.Ullrich E, Bonmort M, Mignot G, et al. Trans-Presentation of IL-15 Dictates IFN-Producing Killer Dendritic Cells Effector Functions. J Immunol. 2008;180:7887–7897. [Google Scholar]
- 79.Mignot G, Ullrich E, Bonmort M, et al. The Critical Role of IL-15 in the Antitumor Effects Mediated by the Combination Therapy Imatinib and IL-2. J Immunol. 2008;180:6477–6483. doi: 10.4049/jimmunol.180.10.6477. [DOI] [PubMed] [Google Scholar]
- 80.Nakazato K, Yamada H, Yajima T, Kagimoto Y, Kuwano H, Yoshikai Y. Enforced Expression of Bcl-2 Partially Restores Cell Numbers but Not Functions of TCR{gamma}{delta} Intestinal Intraepithelial T Lymphocytes in IL-15-Deficient Mice. J Immunol. 2007;178:757–764. doi: 10.4049/jimmunol.178.2.757. [DOI] [PubMed] [Google Scholar]
- 81.Zhao H, Nguyen H, Kang J. Interleukin 15 controls the generation of the restricted T cell receptor repertoire of [gamma][delta] intestinal intraepithelial lymphocytes. Nat Immunol. 2005;6:1263–1271. doi: 10.1038/ni1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gill N, Paltser G, Ashkar AA. Interleukin-15 expression affects homeostasis and function of B cells through NK cell-derived interferon-[gamma] Cellular Immunology. 2009 doi: 10.1016/j.cellimm.2009.03.010. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 83.Abdel-Salam BKA-H, Ebaid H. Upregulation of major histocompatibility complex class II, CD83, CD64, and CD14 on polymorphonuclear neutrophils stimulated with interleukin-15. J Microbiol Immunol Infect. 2008;41:462–468. [PubMed] [Google Scholar]
- 84.Brilot F, Strowig T, Roberts SM, Arrey F, Münz C. NK cell survival mediated through the regulatory synapse with human DCs requires IL-15Ralpha. The journal of clinical investigation. 2007;117:3316–3329. doi: 10.1172/JCI31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic Cells Prime Natural Killer Cells by trans-Presenting Interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fujisaki H, Kakuda H, Shimasaki N, et al. Expansion of Highly Cytotoxic Human Natural Killer Cells for Cancer Cell Therapy. Cancer Res. 2009;69:4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujisaki H, Kakuda H, Imai C, Mullighan C, Campana D. Replicative potential of human natural killer cells. British Journal of Haematology. 2009;9999 doi: 10.1111/j.1365-2141.2009.07667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Numbenjapon T, Serrano LM, Chang W-C, Forman SJ, Jensen MC, Cooper LJN. Antigen-independent and antigen-dependent methods to numerically expand CD19-specific CD8+ T cells. Experimental Hematology. 2007;35:1083–1090. doi: 10.1016/j.exphem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 89.Tang F, Zhao LT, Jiang Y, Ba DN, Cui LX, He W. Activity of recombinant human interleukin-15 against tumor recurrence and metastasis in mice. Cellular & molecular immunology. 2008;5:189–196. doi: 10.1038/cmi.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang M, Yao Z, Dubois S, Ju W, Muller R, Jr, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proceedings of the National Academy of Sciences. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vera M, Razquin N, Prieto J, Melero I, Fortes P, Gonzalez-Aseguinolaza G. Intratumoral Injection of Dendritic Cells Transduced by an SV40-Based Vector Expressing Interleukin-15 Induces Curative Immunity Mediated by CD8+ T Lymphocytes and NK Cells. Mol Ther. 2005;12:950–959. doi: 10.1016/j.ymthe.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 92.Sasahira T, Sasaki T, Kuniyasu H. Interleukin-15 and transforming growth factor alpha are associated with depletion of tumor-associated macrophages in colon cancer. Journal of experimental & clinical cancer research. 2005;24:69–74. [PubMed] [Google Scholar]
- 93.Ugen KE, Kutzler MA, Marrero B, et al. Regression of subcutaneous B16 melanoma tumors after intratumoral delivery of an IL-15-expressing plasmid followed by in vivo electroporation. Cancer Gene Ther. 2006;13:969–974. doi: 10.1038/sj.cgt.7700973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Epardaud M, Elpek KG, Rubinstein MP, et al. Interleukin-15/Interleukin-15R{alpha} Complexes Promote Destruction of Established Tumors by Reviving Tumor-Resident CD8+ T Cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 95.Le H, Graham L, Miller C, Kmieciak M, Manjili M, Bear H. Incubation of antigen-sensitized T lymphocytes activated with bryostatin 1 + ionomycin in IL-7 + IL-15 increases yield of cells capable of inducing regression of melanoma metastases compared to culture in IL-2. Cancer Immunology, Immunotherapy. 2009 doi: 10.1007/s00262-009-0666-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Basak GW, Zapala L, Wysocki PJ, Mackiewicz A, Jakobisiak M, Lasek W. Interleukin 15 augments antitumor activity of cytokine gene-modified melanoma cell vaccines in a murine model. Oncology reports. 2008;19:1173–1179. [PubMed] [Google Scholar]
- 97.Verhoeven DHJ, de Hooge ASK, Mooiman ECK, et al. NK cells recognize and lyse Ewing sarcoma cells through NKG2D and DNAM-1 receptor dependent pathways. Molecular Immunology. 2008;45:3917–3925. doi: 10.1016/j.molimm.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 98.Castriconi R, Daga A, Dondero A, et al. NK Cells Recognize and Kill Human Glioblastoma Cells with Stem Cell-Like Properties. J Immunol. 2009;182:3530–3539. doi: 10.4049/jimmunol.0802845. [DOI] [PubMed] [Google Scholar]
- 99.King JW, Thomas S, Corsi F, et al. IL15 Can Reverse the Unresponsiveness of Wilms’ Tumor Antigen-Specific CTL in Patients with Prostate Cancer. Clin Cancer Res. 2009;15:1145–1154. doi: 10.1158/1078-0432.CCR-08-1821. [DOI] [PubMed] [Google Scholar]
- 100.Teague RM, Sather BD, Sacks JA, et al. Interleukin-15 rescues tolerant CD8+ T cells for use in adoptive immunotherapy of established tumors. Nat Med. 2006;12:335–341. doi: 10.1038/nm1359. [DOI] [PubMed] [Google Scholar]
- 101.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. The journal of clinical investigation. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boyiadzis M, Memon S, Carson J, et al. Up-regulation of NK Cell Activating Receptors Following Allogeneic Hematopoietic Stem Cell Transplantation under a Lymphodepleting Reduced Intensity Regimen is Associated with Elevated IL-15 Levels. Biology of Blood and Marrow Transplantation. 2008;14:290–300. doi: 10.1016/j.bbmt.2007.12.490. [DOI] [PubMed] [Google Scholar]
- 103.Chen G, Wu D, Wang Y, et al. Expanded donor natural killer cell and IL-2, IL-15 treatment efficacy in allogeneic hematopoietic stem cell transplantation. European Journal of Haematology. 2008;81:226–235. doi: 10.1111/j.1600-0609.2008.01108.x. [DOI] [PubMed] [Google Scholar]
- 104.Alpdogan O, Eng JM, Muriglan SJ, et al. Interleukin-15 enhances immune reconstitution after allogeneic bone marrow transplantation. Blood. 2005;105:865–873. doi: 10.1182/blood-2003-09-3344. [DOI] [PubMed] [Google Scholar]
- 105.Zambello R, Facco M, Trentin L, et al. Interleukin-15 Triggers the Proliferation and Cytotoxicity of Granular Lymphocytes in Patients With Lymphoproliferative Disease of Granular Lymphocytes. Blood. 1997;89:201–211. [PubMed] [Google Scholar]
- 106.Zhang R, Shah MV, Yang J, et al. Network model of survival signaling in large granular lymphocyte leukemia. Proceedings of the National Academy of Sciences. 2008;105:16308–16313. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Totero D, Meazza R, Capaia M, et al. The opposite effects of IL-15 and IL-21 on CLL B cells correlate with differential activation of the JAK/STAT and ERK1/2 pathways. Blood. 2008;111:517–524. doi: 10.1182/blood-2007-04-087882. [DOI] [PubMed] [Google Scholar]
- 108.Badoual C, Bouchaud G, Agueznay NEH, et al. The Soluble {alpha} Chain of Interleukin-15 Receptor: A Proinflammatory Molecule Associated with Tumor Progression in Head and Neck Cancer. Cancer Res. 2008;68:3907–3914. doi: 10.1158/0008-5472.CAN-07-6842. [DOI] [PubMed] [Google Scholar]
- 109.Tejman-Yarden N, Zlotnik M, Lewis E, Etzion O, Chaimovitz C, Douvdevani A. Renal cells express a functional interleukin-15 receptor. Nephrology dialysis transplantation. 2005;20:516–523. doi: 10.1093/ndt/gfh616. [DOI] [PubMed] [Google Scholar]
- 110.Khawam K, Giron-Michel J, Gu Y, et al. Human Renal Cancer Cells Express a Novel Membrane-Bound Interleukin-15 that Induces, in Response to the Soluble Interleukin-15 Receptor {alpha} Chain, Epithelial-to-Mesenchymal Transition. Cancer Res. 2009;69:1561–1569. doi: 10.1158/0008-5472.CAN-08-3198. [DOI] [PubMed] [Google Scholar]
- 111.Cario G, Izraeli S, Teichert A, et al. High Interleukin-15 Expression Characterizes Childhood Acute Lymphoblastic Leukemia With Involvement of the CNS. J Clin Oncol. 2007;25:4813–4820. doi: 10.1200/JCO.2007.11.8166. [DOI] [PubMed] [Google Scholar]
- 112.Wu S, Ge ner R, von Stackelberg A, Kirchner R, Henze G, Seeger K. Cytokine/cytokine receptor gene expression in childhood acute lymphoblastic leukemia. Cancer. 2005;103:1054–1063. doi: 10.1002/cncr.20869. [DOI] [PubMed] [Google Scholar]
- 113.Fehniger TA, Suzuki K, Ponnappan A, et al. Fatal Leukemia in Interleukin 15 Transgenic Mice Follows Early Expansions in Natural Killer and Memory Phenotype CD8+ T Cells. J Exp Med. 2001;193:219–232. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee C, Lin S, Cheng P, Kuo M. The regulatory function of umbilical cord blood CD4+CD25+ T cells stimulated with anti-CD3/anti-CD28 and exogenous interleukin (IL)-2 or IL-15. Pediatric Allergy and Immunology. 2009:9999. doi: 10.1111/j.1399-3038.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 115.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but Not Thymic Stromal Lymphopoeitin, Redundantly Govern CD4+Foxp3+ Regulatory T Cell Development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Imamichi H, Sereti I, Lane HC. IL-15 acts as a potent inducer of CD4(+)CD25(hi) cells expressing FOXP3. European Journal of Immunology. 2008;38:1621–1630. doi: 10.1002/eji.200737607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blaser BW, Schwind NR, Karol S, et al. Trans-presentation of donor-derived interleukin 15 is necessary for the rapid onset of acute graft-versus-host disease but not for graft-versus-tumor activity. Blood. 2006;108:2463–2469. doi: 10.1182/blood-2006-04-019059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Blaser BW, Roychowdhury S, Kim DJ, et al. Donor-derived IL-15 is critical for acute allogeneic graft-versus-host disease. Blood. 2005;105:894–901. doi: 10.1182/blood-2004-05-1687. [DOI] [PubMed] [Google Scholar]
- 119.Roychowdhury S, Blaser BW, Freud AG, et al. IL-15 but not IL-2 rapidly induces lethal xenogeneic graft-versus-host disease. Blood. 2005;106:2433–2435. doi: 10.1182/blood-2005-04-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–8731. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 121.Asao H, Okuyama C, Kumaki S, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 122.Wurster AL, Rodgers VL, Satoskar AR, et al. Interleukin 21 is a T helper (Th) cell 2 cytokine that specifically inhibits the differentiation of naive Th cells into interferon gamma-producing Th1 cells. J Exp Med. 2002;196:969–977. doi: 10.1084/jem.20020620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mehta DS, Wurster AL, Weinmann AS, Grusby MJ. NFATc2 and T-bet contribute to T-helper-cell-subset-specific regulation of IL-21 expression. Proc Natl Acad Sci U S A. 2005;102:2016–2021. doi: 10.1073/pnas.0409512102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skak K, Frederiksen K, Lundsgaard D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology. 2008;123:575–583. doi: 10.1111/j.1365-2567.2007.02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takaki R, Hayakawa Y, Nelson A, et al. IL-21 Enhances Tumor Rejection through a NKG2D-Dependent Mechanism. J Immunol. 2005;175:2167–2173. doi: 10.4049/jimmunol.175.4.2167. [DOI] [PubMed] [Google Scholar]
- 126.Wang G, Tschoi M, Spolski R, et al. In vivo antitumor activity of interleukin 21 mediated by natural killer cells. Cancer Res. 2003;63:9016–9022. [PubMed] [Google Scholar]
- 127.Kasaian MT, Whitters MJ, Carter LL, et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity. 2002;16:559–569. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 128.Parrish-Novak J, Dillon SR, Nelson A, et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature. 2000;408:57–63. doi: 10.1038/35040504. [DOI] [PubMed] [Google Scholar]
- 129.Zeng R, Spolski R, Finkelstein SE, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van Leeuwen EM, Gamadia LE, Baars PA, Remmerswaal EB, ten Berge IJ, van Lier RA. Proliferation requirements of cytomegalovirus-specific, effector-type human CD8+ T cells. J Immunol. 2002;169:5838–5843. doi: 10.4049/jimmunol.169.10.5838. [DOI] [PubMed] [Google Scholar]
- 131.Mehta DS, Wurster AL, Whitters MJ, Young DA, Collins M, Grusby MJ. IL-21 induces the apoptosis of resting and activated primary B cells. J Immunol. 2003;170:4111–4118. doi: 10.4049/jimmunol.170.8.4111. [DOI] [PubMed] [Google Scholar]
- 132.Spolski R, Kim H-P, Zhu W, Levy DE, Leonard WJ. IL-21 Mediates Suppressive Effects via Its Induction of IL-10. J Immunol. 2009;182:2859–2867. doi: 10.4049/jimmunol.0802978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thompson JA, Curti BD, Redman BG, et al. Phase I Study of Recombinant Interleukin-21 in Patients With Metastatic Melanoma and Renal Cell Carcinoma. J Clin Oncol. 2008;26:2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 134.Davis ID, Skrumsager BK, Cebon J, et al. An Open-Label, Two-Arm, Phase I Trial of Recombinant Human Interleukin-21 in Patients with Metastatic Melanoma. Clin Cancer Res. 2007;13:3630–3636. doi: 10.1158/1078-0432.CCR-07-0410. [DOI] [PubMed] [Google Scholar]
- 135.Davis ID, Brady B, Kefford RF, et al. Clinical and Biological Efficacy of Recombinant Human Interleukin-21 in Patients with Stage IV Malignant Melanoma without Prior Treatment: A Phase IIa Trial. Clin Cancer Res. 2009;15:2123–2129. doi: 10.1158/1078-0432.CCR-08-2663. [DOI] [PubMed] [Google Scholar]
- 136.Dodds M, Frederiksen K, Skak K, et al. Immune activation in advanced cancer patients treated with recombinant IL-21: multianalyte profiling of serum proteins. Cancer Immunology, Immunotherapy. 2009;58:843–854. doi: 10.1007/s00262-008-0600-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Frederiksen K, Lundsgaard D, Freeman J, et al. IL-21 induces in vivo immune activation of NK cells and CD8+ T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunology, Immunotherapy. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Totero D, Meazza R, Zupo S, et al. Interleukin-21 receptor (IL-21R) is up-regulated by CD40 triggering and mediates proapoptotic signals in chronic lymphocytic leukemia B cells. Blood. 2006;107:3708–3715. doi: 10.1182/blood-2005-09-3535. [DOI] [PubMed] [Google Scholar]
- 139.Gowda A, Roda J, Hussain S-RA, et al. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood. 2008;111:4723–4730. doi: 10.1182/blood-2007-07-099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Akamatsu N, Yamada Y, Hasegawa H, et al. High IL-21 receptor expression and apoptosis induction by IL-21 in follicular lymphoma. Cancer Letters. 2007;256:196–206. doi: 10.1016/j.canlet.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 141.Fang L, Wang K, Liu X, et al. A study on anti-tumor immunity induced by gene-modified melanoma B16 cells. Oncology reports. 2008;19:1589–1595. [PubMed] [Google Scholar]
- 142.Hinrichs CS, Spolski R, Paulos CM, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Dou J, Chu L, Zhao F, et al. Study of immunotherapy of murine myeloma by an IL-21-based tumor vaccine in BALB/C mice. Cancer biology & therapy. 2007;6:1871–1879. doi: 10.4161/cbt.6.12.4960. [DOI] [PubMed] [Google Scholar]
- 144.Kumano M, Hara I, Furukawa J, et al. Interleukin-21 Activates Cytotoxic T Lymphocytes and Natural Killer Cells to Generate Antitumor Response in Mouse Renal Cell Carcinoma. The Journal of Urology. 2007;178:1504–1509. doi: 10.1016/j.juro.2007.05.115. [DOI] [PubMed] [Google Scholar]
- 145.Furukawa J, Hara I, Nagai H, Yao A, Oniki S, Fujisawa M. Interleukin-21 Gene Transfection Into Mouse Bladder Cancer Cells Results in Tumor Rejection Through the Cytotoxic T Lymphocyte Response. The Journal of Urology. 2006;176:1198–1203. doi: 10.1016/j.juro.2006.04.037. [DOI] [PubMed] [Google Scholar]
- 146.Daga A, Orengo A, Maria R, et al. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. International Journal of Cancer. 2007;121:1756–1763. doi: 10.1002/ijc.22901. [DOI] [PubMed] [Google Scholar]
- 147.Kim-Schulze S, Kim HS, Fan Q, Kim DW, Kaufman HL. Local IL-21 Promotes the Therapeutic Activity of Effector T cells by Decreasing Regulatory T Cells Within the Tumor Microenvironment. Mol Ther. 2008;17:380–388. doi: 10.1038/mt.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thedrez A, Harly C, Morice A, Salot S, Bonneville M, Scotet E. IL-21-Mediated Potentiation of Antitumor Cytolytic and Proinflammatory Responses of Human V{gamma}9V{delta}2 T Cells for Adoptive Immunotherapy. J Immunol. 2009;182:3423–3431. doi: 10.4049/jimmunol.0803068. [DOI] [PubMed] [Google Scholar]
- 149.Iuchi T, Teitz-Tennenbaum S, Huang J, et al. Interleukin-21 Augments the Efficacy of T-Cell Therapy by Eliciting Concurrent Cellular and Humoral Responses. Cancer Res. 2008;68:4431–4441. doi: 10.1158/0008-5472.CAN-07-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eriksen KW, Søndergaard H, Woetmann A, et al. The combination of IL-21 and IFN-[alpha] boosts STAT3 activation, cytotoxicity and experimental tumor therapy. Molecular Immunology. 2009;46:812–820. doi: 10.1016/j.molimm.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 151.Liu S, Lizee G, Lou Y, et al. IL-21 synergizes with IL-7 to augment expansion and anti-tumor function of cytotoxic T cells. Int Immunol. 2007;19:1213–1221. doi: 10.1093/intimm/dxm093. [DOI] [PubMed] [Google Scholar]
- 152.Smyth MJ, Teng MWL, Sharkey J, et al. Interleukin 21 Enhances Antibody-Mediated Tumor Rejection. Cancer Res. 2008;68:3019–3025. doi: 10.1158/0008-5472.CAN-07-6019. [DOI] [PubMed] [Google Scholar]
- 153.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE., III Interleukin-21 Enhances NK Cell Activation in Response to Antibody-Coated Targets. J Immunol. 2006;177:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 154.Croce M, Meazza R, Orengo A, et al. Immunotherapy of neuroblastoma by an Interleukin-21-secreting cell vaccine involves survivin as antigen. Cancer Immunology, Immunotherapy. 2008;57:1625–1634. doi: 10.1007/s00262-008-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Søndergaard H, Frederiksen K, Thygesen P, et al. Interleukin 21 therapy increases the density of tumor infiltrating CD8+ T cells and inhibits the growth of syngeneic tumors. Cancer Immunology, Immunotherapy. 2007;56:1417–1428. doi: 10.1007/s00262-007-0285-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lamprecht B, Kreher S, Anagnostopoulos I, et al. Aberrant expression of the Th2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3{alpha} Blood. 2008;112:3339–3347. doi: 10.1182/blood-2008-01-134783. [DOI] [PubMed] [Google Scholar]
- 157.Scheeren FA, Diehl SA, Smit LA, et al. IL-21 is expressed in Hodgkin lymphoma and activates STAT5: evidence that activated STAT5 is required for Hodgkin lymphomagenesis. Blood. 2008;111:4706–4715. doi: 10.1182/blood-2007-08-105643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Menoret E, Maiga S, Descamps G, et al. IL-21 Stimulates Human Myeloma Cell Growth through an Autocrine IGF-1 Loop. J Immunol. 2008;181:6837–6842. doi: 10.4049/jimmunol.181.10.6837. [DOI] [PubMed] [Google Scholar]
- 159.Ettinger R, Kuchen S, Lipsky PE. Interleukin 21 as a target of intervention in autoimmune disease. Ann Rheum Dis. 2008;67:iii83–86. doi: 10.1136/ard.2008.098400. [DOI] [PubMed] [Google Scholar]


