Abstract
PPARγ activation in type 2 diabetic patients results in a marked improvement in insulin and glucose parameters, resulting from an improvement of whole-body insulin sensitivity. Adipose tissue is the major mediator of PPARγ action on insulin sensitivity. PPARγ activation in mature adipocytes induces the expression of a number of genes involved in the insulin signaling cascade, thereby improving insulin sensitivity. PPARγ is the master regulator of adipogenesis, thereby stimulating the production of small insulin-sensitive adipocytes. In addition to its importance in adipogenesis, PPARγ plays an important role in regulating lipid, metabolism in mature adipocytes by increasing fatty acid trapping. Finally, adipose tissue produces several cytokines that regulate energy homeostasis, lipid and glucose metabolism. Disturbances in the production of these factors may contribute to metabolic abnormalities, and PPARγ activation is also associated with beneficial effects on expression and secretion of a whole range of cytokines.
1. Introduction
As a major tissue for whole-body energy homeostasis, adipose tissue integrates both central and peripheral metabolic signals that orchestrate energy balance. An imbalance between energy intake and energy expenditure leads to the expansion of adipose tissue, characterized by a combination of cell proliferation (hyperplasia) and cell size increase (hypertrophy). A complex and yet incompletely defined series of transcriptional events represents the fundamental biological mechanism through which multipotent mesenchymal precursor cells become committed to the adipocyte lineage and exhibit the typical markers of mature fat cells. Identifying the mechanisms that control differentiation of adipose cells would provide clues for designing comprehensive therapeutic strategies for the prevention and treatment of adipose tissue expansion and its associated clinical disorders, including hyperlipemia, hypertension, and type 2 diabetes. However, the mechanisms that regulate adipose cell number and size during adipogenesis are still poorly understood.
In recent years, it has become evident that the societies of the developed countries are at immense risk of metabolic diseases, the so-called civilization diseases or X syndrome. In fact, the rise in the prevalence of specific endocrine-related diseases such as obesity and diabetes clearly suggests an importance of either environmental or genetic factors. The therapy of metabolic diseases assumes the recognition and detailed understanding of the molecular events that control these disorders as well as the development of therapeutics targeting the responsible factors. Recently, several different transcriptional factors have been identified as regulators of the expression of a set of genes involved in glucose and lipid metabolism. Among them, peroxisome proliferator-activated receptors (PPARs), belonging to the superfamily of nuclear receptors (NRs), have been shown to play a central role in the transcriptional control of genes encoding proteins involved in the above processes.
2. PPAR Nuclear Receptors
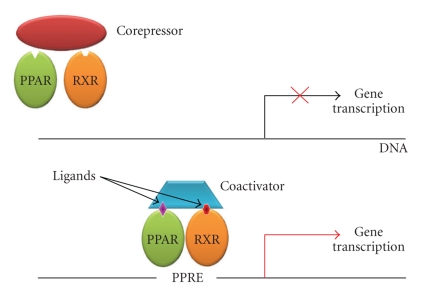
Peroxisome proliferator-activated receptors (PPARs) exist as an obligate heterodimer with the retinoic X receptor (RXR) [1] and are localized to the nucleus also in the unligated state [2]. Upon ligand binding, a conformational change leads to corepressor release and coactivator binding. The binding pocket permits binding of ligands with quite diverse structures [3], probably resulting in different conformational changes which, in turn, affect the recruitment of cofactors and regulate the kinetics of the assembly of the transcription complex, as well as the affinity for the specific PPAR response element (PPRE). The PPAR/RXR heterodimers can be activated by ligands of either receptor, and simultaneous binding of both ligands has been shown to be more efficient in some cases [4]. After ligand binding and activation, the heterodimers are able to either enhance or repress gene expression through binding to PPRE in the promoter region of target genes (Figure 1).
Figure 1.
Mechanism of PPARγ activation. Upon ligand binding to the PPAR/RXR heterodimer, a conformational change leads to release of a corepressor and binding of a coactivator; this regulates the kinetics of the assembly of the transcription complex, resulting in increased affinity for the specific PPAR response element, which modulates gene transcription. RXR; Retinoic X receptor; PPRE; PPAR response element.
Three different human PPAR subtypes have been identified so far, designated as PPARα, PPARβ (also known as PPARδ), and PPARγ. Each of them displays a distinct pattern of tissue distribution and a specific role. PPARα is predominantly expressed in the liver and skeletal muscles, participating in fatty-acids catabolism. PPARα also activates fatty-acid oxidation in the kidney, skeletal muscles, and heart [5]. It has been established that PPARβ is present at moderate levels in all human tissues, with a higher expression in the placenta and the large intestine [6]. Very little is known about the functions of PPARβ. However, recent findings have implicated PPARβ as an important regulator of energy expenditure as well as glucose and lipid metabolism [7]. Of the three members of PPARs, PPARγ is the most frequently studied nuclear receptor involved in the control of energy balance and both lipid and glucose homeostasis [8]. PPARγ exists as two protein isoforms, PPARγ1 and γ2, that differ in their N-terminal end as a result of alternative promoter usage [8]. PPARγ1 has a similar expression pattern as PPARα while PPARγ2 is predominantly expressed in adipose tissue where it regulates adipocyte differentiation.
3. Endogenous and Synthetic Ligands
Over the past several years, various natural and synthetic PPARγ ligands, including PPARγ agonists, PPARγ partial agonists, and PPARα/γ dual agonists, have been investigated. Numerous studies have shown that polyunsaturated fatty acids and related molecules can activate PPARγ as well as other PPARs [9–11]. Interestingly, PPARγ responds poorly to native fatty acids compared to PPARα and PPARδ, suggesting that modified fatty acids may be the biological ligands. Certain prostanoids, including 15-deoxy-Δ12,14 prostaglandin J2 (15-dPGJ2), are excellent activators of PPARγ [12, 13]. However, it is unlikely that 15-dPGJ2 is present at sufficient levels in vivo to be a biologically significant ligand. Oxidized fatty acids, such as 9-hydroxy-10,12-octadecadienoic acid and 13-hydroxy-9,11-octadecadienoic acid found in oxidized low-density lipoprotein (LDL), activate PPARγ with increased potency and efficacy relative to native fatty acids and are present at significant concentrations in atherosclerotic lesions [14]. Whether oxidized fatty acids serve as activators in other tissues, however, is not clear. It is possible that different ligands for PPARγ may be of primary importance in other contexts. For example, the ligand responsible for PPARγ activation in adipogenesis may be distinct from those that activate PPARγ in macrophages in the artery wall. Other lipids, such as nitrated fatty acids and lysophosphatidic acid, have also been reported to activate PPARγ [15, 16]. The importance of these molecules in PPARγ biology remains to be established.
The synthetic PPARγ agonists are thought to be factors determining adipocyte differentiation as well as potential antidiabetic drugs [17]. Compounds such as glitazones or thiazolidinediones (TZDs) (pioglitazone and rosiglitazone) are used clinically as insulin sensitizers [18]. They activate PPARγ and decrease insulin resistance and glucose level in the serum of patients with type 2 diabetes [18]. Many drugs belonging to the TZD class exhibit high selectivity for PPARγ and minimal or no activity toward subtypes-α and -β [19]. However, despite significant antidiabetic activities, TZDs may cause several side effects, such as increased adiposity, oedema, and an increased rate of fractures of the small bones of the extremities. From the therapeutic point of view, improvement of the pharmacological profiles of PPARγ ligands is highly required. Therefore, an alternative approach, relying on the identification of partial agonists, was developed. It was recently reported that a PPARγ partial agonist similar to LSN862, that is, (S)-2 methoxy-3-{4-[5-(4-phenoxy)pent-1-ynyl]phenyl}-propionic acid, has better antidiabetic activity and weaker side effects than the TZDs [20]. More recently, a novel family of PPARγ partial agonists (pyrazol-5-yl benzenesulfonamide derivatives) with either high potency or specificity in vitro or glucose-lowering efficacy in vivo has been identified [21]. Interestingly, the X-ray structures of the PPARγ-ligand complexes revealed a lack of hydrogen bonds between them. This is in sharp contrast to PPARγ agonists sharing a common binding mode in which the acidic head groups form a network of hydrogen interactions with His-323, His-449, and Tyr-473 within the ligand binding pocket [22]. Further molecular studies are required to understand how PPARγ partial agonists modulate transcriptional activity through the recruitment of coactivator and corepressor proteins.
Recent discoveries point to ligands that could stimulate more than one isotype of PPAR at similar concentrations. Thus, the insulin-sensitizing effects of PPARγ and the anti-dislipidemic effects of PPARα or β can be simultaneously obtained by using the so-called coligands. PPARα/γ coligands (ragaglitazar, O-arylmandelic acid, LY465608, and KRP-297) have been shown to have better insulin-sensitizing and lipid-lowering potential in diabetic rodents, as compared to standard compounds which can only stimulate one isotype of PPAR [23–25].
4. PPARγ and Insulin Signaling
PPARγ activation through binding of the synthetic TZDs in type 2 diabetic patients results in a marked improvement in whole-body insulin sensitivity, leading to reduced insulin and glucose plasma levels. The mechanisms of PPARγ-mediated insulin sensitization are complex and are thought to involve specific effects on fat, skeletal muscle, and liver, even though adipose tissue appears to be the major target of TZD-mediated effects on insulin sensitivity. At the cellular level, PPARγ activation has been shown to affect the insulin signaling cascade, through direct modulatory effects on the expression and/or phosphorylation of specific signaling molecules.
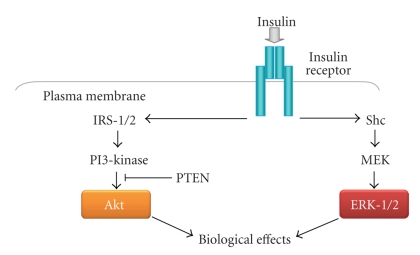
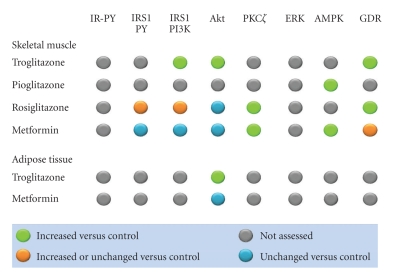
Binding of insulin to its tyrosine kinase receptor engages a cascade of intracellular phosphorylation events, including tyrosine phosphorylation of insulin receptor substrate (IRS) proteins and activation of phosphatidylinositol-3-kinase (PI 3-kinase) and other downstream kinases, which promote multiple biological responses, including glucose uptake, lipid metabolism, survival, differentiation, and modulation of gene transcription (Figure 2). Several groups have shown that PPARγ activation can influence insulin signaling at various steps in these pathways, resulting in improved whole-body insulin sensitivity and enhanced glucose and lipid metabolism. The effects of TZDs on activation of insulin signaling proteins in skeletal muscle and adipose tissue from individuals with type 2 diabetes are summarized in Figure 3.
Figure 2.
Insulin signaling pathway in adipose cells. Binding of insulin to its tyrosine kinase receptor engages a cascade of intracellular phosphorylation events, including activation of phosphatidylinositol-3-kinase and ERK-1/2, that promote multiple biological responses, including glucose uptake, lipid metabolism, survival, differentiation, and modulation of gene transcription.
Figure 3.
Effects of TZDs and metformin on activation of insulin signaling proteins in tissues from individuals with type 2 diabetes. The effects of troglitazone, pioglitazone, and rosiglitazone on various proteins involved in insulin signaling in skeletal muscle and adipose tissue are indicated. The effects of metformin are also shown for comparison. GDR indicates the glucose disposal rate, as a measure of insulin sensitivity. IR-PY: insulin receptor tyrosine phosphorylation; IRS1: insulin receptor substrate-1; PY: tyrosine phosphorylation; PI3K: phosphatidylinositol 3 kinase. Adapted from [26–33].
4.1. IRS Proteins
The IRSs are a large family of docking proteins that act as an interface between the insulin receptor and a complex network of intracellular-signaling molecules. Hammarstedt et al. [34] observed no change in the expression of multiple insulin signaling molecules, including IRS-1, in adipose tissue of pioglitazone-treated nonobese, insulin-resistant individuals [35]. However, a number of studies have demonstrated modulatory effects of TZDs on IRS phosphorylation. In both HEK-293 cells overexpressing a recombinant IRS-1 protein and 3T3-L1 adipocytes, rosiglitazone reduces the PMA-induced inhibitory serine phosphorylation of IRS-1 and restores downstream insulin signaling [36]. The increased levels of IRS-1 serine phosphorylation seen in adipose cells of obese Zucker rats were also found to be reduced after TZD treatment. TZDs may act primarily by reducing the circulating levels of FFA, which have been shown to induce serine phosphorylation of IRS-1 through activation of the protein kinase C isoform PKCθ [37]. In obese Zucker rats, short-term treatment with both rosiglitazone and a non-TZD PPARγ ligand could potentiate the insulin effect and increase the tyrosine phosphorylation of the insulin receptor and IRS-1 as well as induce activation of Akt/PKB [38]. Effects of PPARγ activation have also been reported on IRS-2: in both cultured human adipocytes and 3T3-L1 adipocytes, IRS-2 was found to be increased, both at the gene and protein level, after pioglitazone treatment [39].
4.2. The PI 3-Kinase/Akt Pathway
PI-3 kinase acts as a critical signaling molecule triggering a number of insulin-stimulated effects, including glucose uptake, glycogen synthesis, and cell differentiation. Multiple clinical studies have investigated the effects of TZDs on glucose disposal rates and the insulin signal transduction system in type 2 diabetic patients. TZDs, particularly troglitazone and rosiglitazone, were found to markedly improve glucose disposal rates [26, 27], whereas the effects of metformin appeared less prominent [28, 29]. Studies in which biopsies of subcutaneous abdominal adipose tissue of diabetic patients were taken before and after a period of therapy with either metformin or troglitazone showed no significant effects on total cellular levels of p85, p110β, or Akt proteins with either treatment; however, the insulin effect on Akt phosphorylation was increased with troglitazone, while it was unaltered after metformin treatment [30]. The effects of TZDs on insulin signaling molecules have also been investigated in human skeletal muscle. Treatment with troglitazone increased insulin-stimulated IRS-1-associated PI 3-kinase activity and Akt activity in skeletal muscle biopsies from type 2 diabetic patients [26] and enhanced Akt phosphorylation in skeletal muscle from glucose-tolerant, insulin-resistant, first-degree relatives of type 2 diabetic patients [31]. More controversial appear to be the effects of rosiglitazone on PI 3-kinase activity and Akt phosphorylation. While Miyazaki et al. showed that the improvement in insulin-stimulated muscle glucose disposal after rosiglitazone therapy was associated with increased IRS-1 tyrosine phosphorylation and IRS-1-associated PI 3-kinase activity [32], Karlsson et al. found no changes in IRS-1/PI 3-kinase and Akt/AS160 signaling in patients with newly diagnosed type 2 diabetes, thus concluding that the insulin-sensitizing effects of rosiglitazone were independent of enhanced insulin signaling via these proteins [28]. Interestingly, no effect of metformin therapy on PI 3-kinase or Akt activation in diabetic muscle has been documented [26, 29].
4.3. 5′-AMP-Activated Protein Kinase (5′-AMP Kinase)
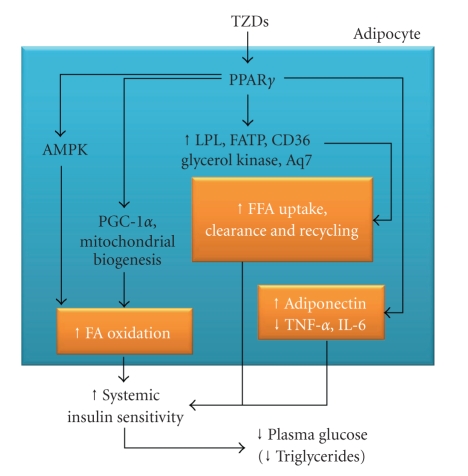
5′-AMP kinase is a key regulator of both glucose and lipid metabolism, which is associated with improved insulin signaling and enhanced insulin sensitivity in skeletal muscle. 5′-AMP kinase activation increases fatty acid oxidation in skeletal muscle by decreasing malonyl CoA concentrations. Both TZDs (i.e., pioglitazone) [33] and metformin [29] have been shown to improve glucose tolerance via adenosine 5′-AMP kinase. Activation of AMPK by metformin decreased the level of plasma glucose and plasma triglycerides by promoting muscle glucose uptake and inhibiting hepatic glucose output [40]. Recently, Coletta et al. have demonstrated that pioglitazone activates 5′-AMP kinase and acetyl-CoA carboxylase (ACC) in human muscle biopsies from patients with type 2 diabetes, leading to increased expression of genes involved in mitochondrial function and fat oxidation, and reduced toxic burden of intracellular lipid metabolites (fatty acyl CoA, diacylglycerol, ceramides) [33] (Figure 4).
Figure 4.
Cellular effects of PPARγ activation in adipocytes. TZDs improve whole-body insulin sensitivity by modulating glucose and lipid metabolism in adipose tissue as well as adipokine secretion by adipocytes. FA: fatty acids.
4.4. ERK-1/2
The ERK proteins, which belong to the family of MAP kinases, modulate cellular responses to environmental stress, cell survival, proliferation, and differentiation. Transfection of cultured cells with a dominant negative MEK, which is the ERK activating kinase, results in decreased effects of both insulin and TZDs on PPARγ activity, suggesting that ERK is involved in the cross-talk between insulin and PPARγ [41]. In vitro assays demonstrate that both ERK2 and JNK are able to phosphorylate PPARγ2 [42]. The MAPK phosphorylation site, which can be used by both ERK- and JNK-MAPK [43], was mapped at serine 82 of mouse PPARγ1, which corresponds to serine 112 of mouse PPARγ2 [44]. Substitution of this serine by alanine (S82A) leads to a loss of PDGF-mediated repression of PPARγ activity [45]. Human PPARγ1 phosphorylation at this site (S84) inhibits both its ligand-dependent and ligand-independent transactivating function. The S84A mutant showed an increase in the AF-1 transcriptional activity of PPARγ [46]. Treatment of macrophages with TGFβ1 increases PPARγ phosphorylation and decreases TZD-induced CD36 expression via activation of the ERK-MAPK pathway [47]. Mutation of the main MAPK site of phosphorylation in PPARγ2 (S112D) results in a decreased ligand-binding affinity [41]. Limited protease digestion shows that the unliganded PPARγ2 and the S112D mutant have different sensitivity; thus, the phosphorylation status of serine 112 plays a role in the conformation of the unliganded receptor which regulates the affinity of PPARγ for its ligands and affects its coactivator recruitment ability [44]. It has been proposed that phosphorylation-mediated inhibition of transcriptional activity of nuclear receptors is an important “off-switch” of ligand-induced activity (reviewed in [48]). Extracellular signals which activate intracellular phosphorylation pathways can also influence the degradation process of PPARγ [49]. As an example, treatment of cells with an MEK inhibitor blocks the degradation of PPARγ. However, not all phosphorylation events are inhibitory and enhance proteosomal degradation, and thus care must be taken when making a global speculation. Substitution of proline to glutamine at position 115 renders PPARγ constitutively active through the modulation of the MAPK-dependent phosphorylation status of serine 114 [50]. Subjects carrying this mutation are extremely obese but surprisingly show a lesser insulin resistance than expected. Mice homozygous for the S112A mutant (homologous to human S114) [51] are protected against diet-induced obesity. This may be due to changes in adipocyte function, such as secretion of adiponectin and leptin. Overall, prevention of PPARγ phosphorylation leads to an improvement of insulin sensitivity mainly due to increased glucose disposal in muscle, which is similar to TZD treatment [51].
4.5. PPARγ and the Glucose Transport System
PPAR-γ activity has been shown to directly regulate the expression of GLUT4 [52] and c-Cbl associating protein (CAP), both involved in regulating insulin-stimulated glucose transport [53]. The GLUT4 (insulin-dependent) transporter is a key modulator of glucose disposal in both muscle and fat. TZD treatment increased the expression of the insulin-responsive glucose transporter GLUT4. However, in another report of the effect of rosiglitazone on freshly isolated human adipocytes, no effect could be seen on the expression of GLUT4 [54]. In animal models of obesity and diabetes, in which the expression of GLUT4 in adipose cells is reduced, treatment with troglitazone restored its expression to normal levels [55]. Although no complete PPRE has been found in the GLUT4 promoter, PPARγ and its heterodimer partner RXRα have been found to bind and repress the promoter activity of GLUT4. The repression is augmented in the presence of the natural ligand, 15D-prostaglandin J2, but completely alleviated by rosiglitazone [56]. This is a novel mechanism through which a PPARγ ligand can exert an antidiabetic effect, that is, by detaching the PPARγ transcription complex from the promoter, thereby increasing the expression of the target gene. It has also been demonstrated that TZDs increase the expression of CAP either in 3T3-L1 adipocytes and in Zucker (fa/fa) diabetic rats, resulting in the stimulation of glucose transport [57]. The induction of CAP expression by TZDs takes place through direct binding of activated PPAR-γ/RXRα heterodimers to a PPRE in the CAP promoter [53].
Interestingly, experimental deletion of PPARγ results in a decrease in insulin-stimulated glucose transport into 3T3-L1 adipocytes, and this is due to a decrease in GLUT1 and GLUT4 function [58]. It remains to be investigated, however, whether similar direct effects on glucose uptake are also operating in skeletal muscle, where much lower levels of PPARγ expression are observed, but where the majority of glucose disposal occurs. Unfortunately, conflicting findings in the two existing mouse models of muscle-specific PPARγ deletion have so far failed to resolve this issue [59, 60] (see below).
The intracellular protein PTEN (phosphatase and tensin homolog deleted on chromosome 10) has been recently proposed to play a crucial role in the PPARγ-induced regulation of glucose uptake. Kim et al. have demonstrated that the reduction of PTEN expression in skeletal muscle cells and adipocytes may be a primary mechanism of the PPARγ-induced improvement in glucose uptake. Furthermore, decreased PTEN levels, associated with reduced plasma glucose, were observed in adipose and muscle tissues of ob/ob mice treated with two structurally different PPARγ agonists, thus confirming that PPARγ-induced insulin sensitization in vivo is mediated by PTEN downregulation [61].
Several lines of evidence support an emerging role for PPARδ in muscle for glucose and lipid metabolism. The role of PPARδ on whole-body glucose homeostasis has been evaluated in muscle-specific PPARδ transgenic mice [62], which exhibit enzymatic and gene expression profiles that promote oxidative metabolism in skeletal muscle. Moreover, PPARδ transgenic mice have reduced body fat mass due to a reduction of adipose cell size [63]. Given the importance of skeletal muscle insulin resistance in the development of type 2 diabetes and other metabolic diseases, targeted activation of PPARδ in skeletal muscle may represent a novel therapeutical target to enhance glucose metabolism. Indeed, there is evidence that exposure of primary human skeletal muscle cells and C2C12 mouse myotubes to specific PPARδ agonists enhances basal and insulin-stimulated glucose uptake [64].
5. Tissue-Specific PPARγ Effects
5.1. Adipose Tissue
PPARγ has the highest expression levels in adipose tissue compared with other metabolic organs, such as skeletal muscle, liver, and pancreas. PPARγ activation in mature adipocytes induces the expression of a number of genes involved in the insulin signaling cascade, thereby improving insulin sensitivity. PPARγ is the master regulator of adipogenesis, thereby stimulating the production of small insulin-sensitive adipocytes. In addition to its importance in adipogenesis, PPARγ plays an important role in regulating lipid metabolism in mature adipocytes. The induction of adipogenesis associated with the capability for fatty acid trapping has been shown to be an important contributor to the maintenance of systemic insulin sensitivity. Finally, adipose tissue produces several hormones that regulate energy homeostasis, lipid, and glucose metabolism such as leptin, adiponectin, resistin, tumor necrosis factor-α, and others. Disturbances in the production of these factors may contribute to the development of insulin resistance or impaired insulin secretion: PPARγ activation is also associated with beneficial effects on the expression and secretion of a whole range of adipokines.
5.1.1. The Role of PPARγ in Adipogenesis and Differentiation.
Adipogenesis refers to the differentiation process of preadipocyte precursor cells into mature adipocytes during which gene expression, cell morphology, and hormone sensitivity change. Preadipocytes can be differentiated into white (energy storage) and brown (energy dissipation) adipocytes. In the differentiation of white adipocytes, numerous genes encoding proteins participating in fatty-acid metabolism are induced. It is known that the transcription factor PPARγ is an important regulator of the formation of adipose tissue [65–69], since it induces several specific adipose markers, such as adipocyte fatty acid binding protein (aP2) [70], phosphoenolpyruvate carboxykinase (PEPCK) [71], and lipoprotein lipase (LPL) [72]. Moreover, the ectopic expression of PPARγ promotes adipogenesis in nonadipogenic fibroblastic cells such as NIH-3T3 cells [73]. In addition, PPARγ-deficient adipocytes of adult mice die within a few days [73] and PPARγ knockout mice are unable to develop adipose tissue [8]. Consistent with the above, several PPARγ missense mutations (C190S, V290M, F388L, R425C, P467L) in humans are associated with partial lipodystrophy [74]. Although all these studies indicate a pivotal role of PPARγ in adipogenesis, it is likely one of several proteins involved in the regulation of this multifactoral process. Indeed, besides PPARγ, C/EBP transcription factors (C/EBP-α, -β, and -δ) expressed in distinct phases of adipogenesis have been shown to play important roles as well. C/EBP-β and -δ are activated in response to insulin or glucocorticoids in the initial stages of adipogenesis [75, 76] and they, in turn, induce the transcription of PPARγ.
With cell differentiation, mRNA levels of PPARγ are markedly upregulated [77]. In addition, during the early stages of differentiation, another transcriptional factor, namely, ADD1/SREBP1, has been found to affect the transcriptional activity of PPARγ [78]. It has been suggested that this factor can modulate PPARγ activity through the production of endogenous ligands for PPARγ since it participates in the regulation of cholesterol homeostasis and in the expressions of several genes encoding proteins involved in lipid metabolism [75]. In the terminal stages of adipogenesis, PPARγ activates the expression of C/EBP-α; however, C/EBP-α, in response, also induces PPARγ gene expression through binding to the same DNA sites in the PPARγ promoter that are induced by C/EBP-β, and -δ [79]. Thus there is a positive feedback loop between PPARγ and C/EBP-α [80]. The positive cross-regulation between these factors has been observed in C/EBP-α-deficient adipocytes, which accumulate fewer lipids and do not induce endogenous PPARγ [80].
The adipogenic effect of PPARγ activation is likely related to the known effects of glitazones to enhance bone loss and lead to increased risk of bone fractures, which has emerged from clinical trials. Within the bone marrow, the differentiation of the resident mesenchymal stem cells (MSCs) into adipocytes or osteoblasts is competitively balanced, so that mechanisms that promote a given cell fate (i.e., osteogenesis) actively suppress mechanisms that induce the alternative lineage (adipogenesis). Elbrecht et al. [81] first showed that PPARγ is expressed in bone marrow MSCs. Subsequently, it was demonstrated that treatment of bone marrow stromal cells with TZDs resulted in the differentiation of these cells into adipocytes [82] rather than osteoblasts. It has thus been suggested that this unbalanced marrow adipogenesis may contribute to the increased risk of bone fractures in TZD-treated subjects.
In addition to the above transcription factors activating adipogenesis, there are several factors involved in the control of this process, such as tumor necrosis factor- (TNF-) α and leptin. TNF-α is a polypeptide hormone with pleiotropic effects on cellular proliferation and differentiation and is a potent inhibitor of adipogenesis. The exposure of 3T3-L1 adipocytes to TNF-α results in lipid depletion and a complete reversal of adipocyte differentiation [83]. In addition, suppression of several adipocyte genes, such as those encoding aP2, adipsin, and insulin-responsive glucose transporter (GLUT4), has been found [84–86]. This antiadipogenic effect of TNF-α most likely results from the downregulation of C/EBP-α and PPARγ expression [87]. In the case of leptin, which induces lipolysis and glucose utilization in adipocytes, it has been shown that TZD-activated PPARγ inhibits leptin production [88]. This inhibition can be explained in terms of a functional antagonism between C/EBP-α and PPARγ on leptin promoter activity [89].
Apart from adipocyte differentiation, PPARγ activation promotes the apoptosis of mature adipocytes [90]. It has been reported that troglitazone, a PPARγ agonist of the TZD class, increases the population of small adipocytes in white adipose tissue and concomitantly decreases the population of large adipocytes. In addition, the percentage of apoptotic nuclei is increased by 2.5-fold in troglitazone-treated tissues, implying that large adipocytes lost by apoptosis may be counterbalanced by small adipocytes newly differentiated following troglitazone treatment. PPARγ activation by TZD thus leads to the accumulation of small adipocytes, which are more insulin sensitive than the large adipocytes [90].
5.1.2. Modulation of Adipokine Production
Another potential mechanism whereby activation of PPARγ in adipose tissue may impact whole-body insulin sensitivity is by modulating the production of adipokines. Adiponectin is a multimeric plasma protein produced exclusively by adipose tissue that shares homology with the c1q complement protein. Multiple studies have shown that plasma adiponectin levels are inversely correlated with adipose tissue mass and directly correlated with insulin sensitivity [91]. The adiponectin gene is a direct target for regulation by PPARγ [92]. Adiponectin mRNA and plasma protein levels are induced in rodents and humans following TZD administration [93, 94]. Studies in mice have shown that administration of adiponectin leads to suppression of hepatic glucose output and improvement in glucose uptake, reminiscent of the effects of TZDs [95]. Furthermore, mice lacking adiponectin show impaired responses to TZDs [96]. Ligand activation of PPARγ in adipocytes is also associated with decreased production of proteins postulated to cause insulin resistance, including TNF-α and resistin [97]. Knockouts of TNF, TNF receptors, and resistin show improved insulin sensitivity, consistent with a physiological and/or pathophysiological role for these proteins in modulating insulin responses and systemic metabolism [98, 99].
5.2. Skeletal Muscle
The overall improvement of insulin sensitivity which is observed upon glitazone treatment may potentially result from PPARγ activation also in skeletal muscle. Even though PPARγ is expressed at a low level in myofibers of humans and rodents, the net result of skeletal muscle PPARγ activation is potentially relevant, because skeletal muscle is the largest glucose utilizing organ in the body. Mice with genetic deletion of PPARγ in skeletal muscle showed significantly increased whole-body insulin resistance [59, 60, 100], demonstrated either by insulin/glucose tolerance tests or by hyperinsulinemic euglycemic clamp studies, and developed dyslipidaemia, enlarged fat pads, and obesity on high-fat diet [59, 60]. Lipid overload appears to be a primary event in the pathogenesis of insulin resistance, because increased adiposity is observed before the development of overt hyperglycemia or hyperinsulinemia and despite reduced dietary intake [59]. In addition, Hevener et al. [60] postulated that loss of PPARγ resulted in skeletal muscle insulin resistance followed by impaired insulin action in adipose tissue and liver. By contrast, Norris et al. [59] did not observe any change in muscle glucose disposal, whereas hepatic insulin sensitivity was found to be impaired. Regardless of the basis for these conflicting results, it appears that the pharmacological response to TZDs is preserved, at least under some experimental conditions, in mice lacking PPARγ selectively in muscle. Thus, it is unlikely that a direct action on muscle is the primary basis for the clinical effects of PPARγ agonists, again underscoring the importance of adipose tissue as the main mediator of TZD actions [101].
5.3. Liver
In experimental models with ablation of white adipose tissue, hepatic PPARγ participates in both fat regulation and glucose homeostasis, and TZD treatment results in lower triglyceride and glucose levels [102]. However, when adipose tissue is normally expressed, the impact of PPARγ in the liver on glucose homeostasis appears to be minimal. Studies in rodents suggest that activation of hepatic PPARγ signaling promotes lipid accumulation in the liver [102], and hepatic expression of PPARγ is elevated in rodent models of diabetes and insulin resistance that exhibit liver steatosis. Treatment of diabetic mice with TZDs promotes hepatic lipid accumulation, and this effect is abolished in liver-specific PPARγ-null mice [90]. However, expression of PPARγ does not appear to be linked to hepatic steatosis in humans [103]. In fact, studies have suggested that TZDs may be beneficial in treating nonalcolholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) in patients with various degrees of adipose tissue accumulation and metabolic abnormalities [104–106]. However, the ability of PPARγ to directly drive hepatic lipid accumulation in humans treated with TZDs is likely outweighed by the more prominent beneficial effects on fatty acid storage in adipose tissue.
5.4. Systemic Effects
Circulating levels of free fatty acids (FFAs) are a major determinant of insulin sensitivity [107]. The activated PPARγ receptors modulate the expression of genes involved in lipid metabolism and promote fatty acid uptake and storage in adipose tissue. Several studies have shown that the antidiabetic efficacy of TZDs correlates with their ability to lower circulating FFA levels [107]. PPARγ activation by TZDs has been shown to reduce the amount of circulating FFA in the body via adipocyte differentiation and apoptosis. The number of small adipocytes, which are able to accumulate FFA, increases at the expense of hypertrophied adipocytes that release FFA. The distribution of adipose tissue is changed from visceral sites to subcutaneous parts of the body. Thus, PPARγ activation results in more efficient accumulation of fatty acids in the subcutaneous depot [90]. Pharmacological data indicate that PPARγ activation in adipose tissue may exert coordinated effects on FFA flux (promoting uptake/trapping, whilst simultaneously impairing release) through the regulation of a panel of genes involved in FFA metabolism. Adipocyte lipoprotein lipase expression is upregulated in response to TZD treatment, thereby potentially enhancing release of FFAs from circulating lipoproteins [108]. Simultaneous upregulation of FFA transporters such as CD36 and fatty acid transport protein on the adipocyte surface facilitates their uptake [109]. TZDs may also reduce FFA efflux from adipocytes through enhanced expression of genes that promote their storage in the form of triglycerides (e.g. glycerol kinase directs the synthesis of glycerol-3-phosphate directly from glycerol; PEPCK permits the utilization of pyruvate to form the glycerol backbone for triglyceride synthesis) [110, 111]. Coordinated regulation of these pathways ensures that FFAs are stored appropriately in adipose tissue, and not “ectopically” in other sites such as liver and skeletal muscle where they are capable of inducing “lipotoxicity.”
As expected with PPARγ activation, a reduction in plasma FFAs is a consistent observation across many large-scale TZDs clinical trials [112]. This reduction in plasma FFAs also provides a potential mechanism to improve insulin sensitivity in the liver and periphery, as well as reducing lipotoxicity in the pancreatic β-cell and improving insulin secretory function. Accordingly, TZD-induced decreases in NEFA correlate with improvements in both muscle and hepatic insulin sensitivity in patients with type 2 diabetes [113]. A study in PPARγ (−/+) mice showed that PPARγ indirectly protects pancreatic islets from lipotoxicity by regulating triglyceride partitioning among tissues (reducing net influx of NEFA into the islets) and that TZDs can restore insulin secretion impaired by lipotoxicity [114]. It is possible that β-cell protective effects of TZDs may also be mediated indirectly through reduced β-cell stress resulting from the amelioration of insulin resistance. However, based on studies in isolated human islets, there is also evidence that PPARγ activation can have direct effects on β-cell function [115, 116].
6. Conclusions
PPARγ has emerged as a key regulator of adipocyte and macrophage function in adipose tissue. Direct effects of PPARγ activation on adipose tissue lipid metabolism and endocrine function may be linked with secondary benefits in liver and muscle lipid metabolism and insulin signalling and suggest that PPARγ is an important target for pharmacotherapy to tackle the metabolic syndrome and obesity-related insulin resistance. Furthermore, activation of PPARγ in adipose tissue may also have positive effects on pancreatic β-cell function and help to minimize the aggravated paracrine relationship between adipocytes and macrophages seen in obesity. Thus, adipose PPARγ appears to be an essential mediator for the maintenance of whole body insulin sensitivity: protects nonadipose tissues against lipid overload and guarantees appropriate production of adipokines, such as adiponectin and leptin from adipocytes. PPARγ ligands promote the restoration of normal levels of adipose-derived substances, including FFA, TNF-α, leptin, adiponectin, and PAI-1, and reverse major defects of the insulin resistance syndrome due to their important effects on inhibition of atherosclerosis, improvement of endothelial cell function, and attenuation of low-grade inflammation.
Acknowledgments
This work was supported by grants from the Ministero dell'Università e Ricerca (Italy) and a Grant from NovoNordisk (LIBRA Programme) to F. Giorgino. This work was also supported by COST Action BM0602 “Adipose Tissue: a Key Target for Prevention of the Metabolic Syndrome” (EU/ESF).
References
- 1.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes and Development. 1994;8(10):1224–1234. doi: 10.1101/gad.8.10.1224. [DOI] [PubMed] [Google Scholar]
- 2.Berger J, Patel HV, Woods J, et al. A PPARγ mutant serves as a dominant negative inhibitor of PPAR signaling and is localized in the nucleus. Molecular and Cellular Endocrinology. 2000;162(1-2):57–67. doi: 10.1016/s0303-7207(00)00211-2. [DOI] [PubMed] [Google Scholar]
- 3.Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-γ . Nature. 1998;395(6698):137–143. doi: 10.1038/25931. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Rachez C, Freedman LP. Discrete roles for peroxisome proliferator-activated receptor γ and retinoid X receptor in recruiting nuclear receptor coactivators. Molecular and Cellular Biology. 2000;20(21):8008–8017. doi: 10.1128/mcb.20.21.8008-8017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansen MK, Connolly TM. Nuclear receptors as drug targets in obesity, dyslipidemia and atherosclerosis. Current Opinion in Investigational Drugs. 2008;9(3):247–255. [PubMed] [Google Scholar]
- 6.Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacological Research. 2005;51(2):85–94. doi: 10.1016/j.phrs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Billin AN. PPAR-β/δ agonists for type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home. Expert Opinion on Investigational Drugs. 2008;17(10):1465–1471. doi: 10.1517/13543784.17.10.1465. [DOI] [PubMed] [Google Scholar]
- 8.Zieleniak A, Wójcik M, Woźniak LA. Structure and physiological functions of the human peroxisome proliferator-activated receptor γ . Archivum Immunologiae et Therapiae Experimentalis. 2008;56(5):331–345. doi: 10.1007/s00005-008-0037-y. [DOI] [PubMed] [Google Scholar]
- 9.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ . Proceedings of the National Academy of Sciences of the United States of America. 1997;94(9):4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krey G, Braissant O, L’Horset F, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Molecular Endocrinology. 1997;11(6):779–791. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 12.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-deoxy-Δ12,14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ . Cell. 1995;83(5):803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 13.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor γ and promotes adipocyte differentiation. Cell. 1995;83(5):813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 14.Nagy L, Tontonoz P, Alvarez JGA, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ . Cell. 1998;93(2):229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Baker DL, Yasuda S, et al. Lysophosphatidic acid induces neointima formation through PPARγ activation. Journal of Experimental Medicine. 2004;199(6):763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schopfer FJ, Lin Y, Baker PRS, et al. Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor γ ligand. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(7):2340–2345. doi: 10.1073/pnas.0408384102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. Journal of Biological Chemistry. 1997;272(6):3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 18.Kletzien RF, Clarke SD, Ulrich RG. Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Molecular Pharmacology. 1992;41(2):393–398. [PubMed] [Google Scholar]
- 19.Willson TM, Cobb JE, Cowan DJ, et al. The structure—activity relationship between peroxisome proliferator-activated receptor γ agonism and the antihyperglycemic activity of thiazolidinediones. Journal of Medicinal Chemistry. 1996;39(3):665–668. doi: 10.1021/jm950395a. [DOI] [PubMed] [Google Scholar]
- 20.Reifel-Miller A, Otto K, Hawkins E, et al. A peroxisome proliferator-activated receptor α/γ dual agonist with a unique in vitro profile and potent glucose and lipid effects in rodent models of type 2 diabetes and dyslipidemia. Molecular Endocrinology. 2005;19(6):1593–1605. doi: 10.1210/me.2005-0015. [DOI] [PubMed] [Google Scholar]
- 21.Lu I-L, Huang C-F, Peng Y-H, et al. Structure-based drug design of a novel family of PPARγ partial agonists: virtual screening, X-ray crystallography, and in vitro/in vivo biological activities. Journal of Medicinal Chemistry. 2006;49(9):2703–2712. doi: 10.1021/jm051129s. [DOI] [PubMed] [Google Scholar]
- 22.Xu HE, Lambert MH, Montana VG, et al. Structural determinants of ligand binding selectivity between the peroxisome proliferator-activated receptors. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(24):13919–13924. doi: 10.1073/pnas.241410198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams AD, Hu Z, von Langen D, et al. O-arylmandelic acids as highly selective human PPAR α/γ agonists. Bioorganic and Medicinal Chemistry Letters. 2003;13(19):3185–3190. doi: 10.1016/s0960-894x(03)00702-9. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti R, Vikramadithyan RK, Misra P, et al. Ragaglitazar: a novel PPARα & PPARγ agonist with potent lipid-lowering and insulin-sensitizing efficacy in animal models. British Journal of Pharmacology. 2003;140(3):527–537. doi: 10.1038/sj.bjp.0705463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolf G. The function of the nuclear receptor peroxisome proliferator-activated receptor delta in energy homeostasis. Nutrition Reviews. 2003;61(11):387–390. doi: 10.1301/nr.2003.nov.387-390. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y-B, Ciaraldi TP, Kong A, et al. Troglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110β protein levels in skeletal muscle of type 2 diabetic subjects. Diabetes. 2002;51(2):443–448. doi: 10.2337/diabetes.51.2.443. [DOI] [PubMed] [Google Scholar]
- 27.Beeson M, Sajan MP, Dizon M, et al. Activation of protein kinase C-ζ by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52(8):1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson HKR, Hällsten K, Björnholm M, et al. Effects of metformin and rosiglitazone treatment on insulin signaling and glucose uptake in patients with newly diagnosed type 2 diabetes: a randomized controlled study. Diabetes. 2005;54(5):1459–1467. doi: 10.2337/diabetes.54.5.1459. [DOI] [PubMed] [Google Scholar]
- 29.Musi N, Hirshman MF, Nygren J, et al. Metformin increases AMP-activated protein-kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51(7):2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 30.Ciaraldi TP, Kong APS, Chu NV, et al. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes. 2002;51(1):30–36. doi: 10.2337/diabetes.51.1.30. [DOI] [PubMed] [Google Scholar]
- 31.Meyer MM, Levin K, Grimmsmann T, et al. Troglitazone treatment increases protein kinase B phosphorylation in skeletal muscle of normoglycemic subjects at risk for the development of type 2 diabetes. Diabetes. 2002;51(9):2691–2697. doi: 10.2337/diabetes.51.9.2691. [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki Y, He H, Mandarino LJ, DeFronzo RA. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes. 2003;52(8):1943–1950. doi: 10.2337/diabetes.52.8.1943. [DOI] [PubMed] [Google Scholar]
- 33.Coletta DK, Sriwijitkamol A, Wajcberg E, et al. Pioglitazone stimulates AMP-activated protein kinase signalling and increases the expression of genes involved in adiponectin signalling, mitochondrial function and fat oxidation in human skeletal muscle in vivo: a randomised trial. Diabetologia. 2009;52(4):723–732. doi: 10.1007/s00125-008-1256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammarstedt A, Andersson CX, Rotter Sopasakis V, Smith U. The effect of PPARγ ligands on the adipose tissue in insulin resistance. Prostaglandins Leukotrienes and Essential Fatty Acids. 2005;73(1):65–75. doi: 10.1016/j.plefa.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Hammarstedt A, Sopasakis VR, Gogg S, Jansson P-A, Smith U. Improved insulin sensitivity and adipose tissue dysregulation after short-term treatment with pioglitazone in non-diabetic, insulin-resistant subjects. Diabetologia. 2005;48(1):96–104. doi: 10.1007/s00125-004-1612-3. [DOI] [PubMed] [Google Scholar]
- 36.Jiang G, Dallas-Yang Q, Biswas S, Li Z, Zhang BB. Rosiglitazone, an agonist of peroxisome-proliferator-activated receptor γ (PPARγ), decreases inhibitory serine phosphorylation of IRS1 in vitro and in vivo. Biochemical Journal. 2004;377(2):339–346. doi: 10.1042/BJ20031207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin ME, Marcucci MJ, Cline GW, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 38.Jiang G, Dallas-Yang Q, Li Z, et al. Potentiation of insulin signaling in tissues of Zucker obese rats after acute and long-term treatment with PPARγ agonists. Diabetes. 2002;51(8):2412–2419. doi: 10.2337/diabetes.51.8.2412. [DOI] [PubMed] [Google Scholar]
- 39.Smith U, Gogg S, Johansson A, Olausson T, Rotter V, Svalstedt B. Thiazolidinediones (PPARγ agonists) but not PPARα agonists increase IRS-2 gene expression in 3T3-L1 and human adipocytes. The FASEB Journal. 2001;15(1):215–220. doi: 10.1096/fj.00-0020com. [DOI] [PubMed] [Google Scholar]
- 40.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang B, Berger J, Zhou G, et al. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor γ . Journal of Biological Chemistry. 1996;271(50):31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 42.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 43.Camp HS, Tafuri SR, Leff T. c-jun N-terminal kinase phosphorylates peroxisome proliferator-activated receptor-γ1 and negatively regulates its transcriptional activity. Endocrinology. 1999;140(1):392–397. doi: 10.1210/endo.140.1.6457. [DOI] [PubMed] [Google Scholar]
- 44.Shao D, Rangwala SM, Bailey ST, Krakow SL, Reginato MJ, Lazar MA. Interdomain communication regulating ligand binding by PPAR-γ . Nature. 1998;396(6709):377–380. doi: 10.1038/24634. [DOI] [PubMed] [Google Scholar]
- 45.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. Journal of Biological Chemistry. 1997;272(16):10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 46.Adams M, Reginato MJ, Shao D, Lazar MA, Chatterjee VK. Transcriptional activation by peroxisome proliferator-activated receptor γ is inhibited by phosphorylation at a consensus mitogen-activated protein kinase site. Journal of Biological Chemistry. 1997;272(8):5128–5132. doi: 10.1074/jbc.272.8.5128. [DOI] [PubMed] [Google Scholar]
- 47.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Jr., Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ . Journal of Biological Chemistry. 2000;275(2):1241–1246. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 48.Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cellular Signalling. 2003;15(4):355–366. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 49.Floyd ZE, Stephens JM. Interferon-γ-mediated activation and ubiquitin-proteasome-dependent degradation of PPARγ in adipocytes. Journal of Biological Chemistry. 2002;277(6):4062–4068. doi: 10.1074/jbc.M108473200. [DOI] [PubMed] [Google Scholar]
- 50.Ristow M, Müller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. The New England Journal of Medicine. 1998;339(14):953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 51.Rangwala SM, Rhoades B, Shapiro JS, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Developmental Cell. 2003;5(4):657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 52.Wu Z, Xie Y, Morrison RF, Bucher NLR, Farmer SR. PPARγ induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPα during the conversion of 3T3 fibroblasts into adipocytes. Journal of Clinical Investigation. 1998;101(1):22–32. doi: 10.1172/JCI1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumann CA, Chokshi N, Saltiel AR, Ribon V. Cloning and characterization of a functional peroxisome proliferator activator receptor-γ-responsive element in the promoter of the CAP gene. Journal of Biological Chemistry. 2000;275(13):9131–9135. doi: 10.1074/jbc.275.13.9131. [DOI] [PubMed] [Google Scholar]
- 54.Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor γ with rosiglitazone (BRL 49653) in human adipocytes. Biochemical and Biophysical Research Communications. 1999;265(1):265–271. doi: 10.1006/bbrc.1999.1657. [DOI] [PubMed] [Google Scholar]
- 55.Furuta M, Yano Y, Gabazza EC, et al. Troglitazone improves GLUT4 expression in adipose tissue in an animal model of obese type 2 diabetes mellitus. Diabetes Research and Clinical Practice. 2002;56(3):159–171. doi: 10.1016/s0168-8227(01)00373-4. [DOI] [PubMed] [Google Scholar]
- 56.Armoni M, Kritz N, Harel C, et al. Peroxisome proliferator-activated receptor-γ represses GLUT4 promoter activity in primary adipocytes, and rosiglitazone alleviates this effect. Journal of Biological Chemistry. 2003;278(33):30614–30623. doi: 10.1074/jbc.M304654200. [DOI] [PubMed] [Google Scholar]
- 57.Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferator-activated receptor γ activation stimulates expression of the CAP gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(25):14751–14756. doi: 10.1073/pnas.95.25.14751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liao W, Nguyen MTA, Yoshizaki T, et al. Suppression of PPAR-γ attenuates insulin-stimulated glucose uptake by affecting both GLUT1 and GLUT4 in 3T3-L1 adipocytes. American Journal of Physiology. 2007;293(1):E219–E227. doi: 10.1152/ajpendo.00695.2006. [DOI] [PubMed] [Google Scholar]
- 59.Norris AW, Chen L, Fisher SJ, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. Journal of Clinical Investigation. 2003;112(4):608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hevener AL, He W, Barak Y, et al. Muscle-specific Pparg deletion causes insulin resistance. Nature Medicine. 2003;9(12):1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 61.Kim KY, Cho HS, Jung WH, Kim SS, Cheon HG. Phosphatase and tensin homolog deleted on chromosome 10 suppression is an important process in peroxisome proliferator-activated receptor-β signaling in adipocytes and myotubes. Molecular Pharmacology. 2007;71(6):1554–1562. doi: 10.1124/mol.106.031948. [DOI] [PubMed] [Google Scholar]
- 62.Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor δ controls muscle development and oxidative capability. FASEB Journal. 2003;17(15):2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y-X, Lee C-H, Tiep S, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113(2):159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 64.Krämer DK, Al-Khalili L, Perrini S, et al. Direct activation of glucose transport in primary human myotubes after activation of peroxisome proliferator-activated receptor δ . Diabetes. 2005;54(4):1157–1163. doi: 10.2337/diabetes.54.4.1157. [DOI] [PubMed] [Google Scholar]
- 65.Koutnikova H, Cock T-A, Watanabe M, et al. Compensation by the muscle limits the metabolic consequences of lipodystrophy in PPARγ hypomorphic mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(24):14457–14462. doi: 10.1073/pnas.2336090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jones JR, Barrick C, Kim K-A, et al. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(17):6207–6212. doi: 10.1073/pnas.0306743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) γ: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135(2):798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 69.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 70.Tontonoz P, Nagy L, Alvarez JGA, Thomazy VA, Evans RM. PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93(2):241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 71.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Molecular and Cellular Biology. 1995;15(1):351–357. doi: 10.1128/mcb.15.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schoonjans K, Peinado-Onsurbe J, Lefebvre A-M, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. The EMBO Journal. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 73.Imai T, Takakuwa R, Marchand S, et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gurnell M. Peroxisome proliferator-activated receptor γ and the regulation of adipocyte function: lesssons from human genetic studies. Best Practice and Research: Clinical Endocrinology and Metabolism. 2005;19(4):501–523. doi: 10.1016/j.beem.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 75.Wu Z, Bucher NLR, Farmer SR. Induction of peroxisome proliferator-activated receptor γ during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPβ, C/EBPδ, and glucocorticoids. Molecular and Cellular Biology. 1996;16(8):4128–4136. doi: 10.1128/mcb.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu Z, Xie Y, Bucher NLR, Farmer SR. Conditional ectopic expression of C/EBPβ in NIH-3T3 cells induces PPARγ and stimulates adipogenesis. Genes and Development. 1995;9(19):2350–2363. doi: 10.1101/gad.9.19.2350. [DOI] [PubMed] [Google Scholar]
- 77.Perrini S, Laviola L, Cignarelli A, et al. Fat depot-related differences in gene expression, adiponectin secretion, and insulin action and signalling in human adipocytes differentiated in vitro from precursor stromal cells. Diabetologia. 2008;51(1):155–164. doi: 10.1007/s00125-007-0841-7. [DOI] [PubMed] [Google Scholar]
- 78.Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes and Development. 1996;10(9):1096–1107. doi: 10.1101/gad.10.9.1096. [DOI] [PubMed] [Google Scholar]
- 79.Fajas L, Schoonjans K, Gelman L, et al. Regulation of peroxisome proliferator-activated receptor γ expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Molecular and Cellular Biology. 1999;19(8):5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu Z, Rosen ED, Brun R, et al. Cross-regulation of C/EBPα and PPARγ controls the transcriptional pathway of adipogenesis and insulin sensitivity. Molecular Cell. 1999;3(2):151–158. doi: 10.1016/s1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 81.Elbrecht A, Chen Y, Cullinan CA, et al. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors γ1 and γ2. Biochemical and Biophysical Research Communications. 1996;224(2):431–437. doi: 10.1006/bbrc.1996.1044. [DOI] [PubMed] [Google Scholar]
- 82.Gimble JM, Robinson CE, Wu X, et al. Peroxisome proliferator-activated aeceptor-γ activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Molecular Pharmacology. 1996;50(5):1087–1094. [PubMed] [Google Scholar]
- 83.Torti FM, Dieckmann B, Beutler B. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- 84.Stephens JM, Pekala PH. Transcriptional repression of the C/EBP-α and GLUT4 genes in 3T3-L1 adipocytes by tumor necrosis factor-α. Regulation is coordinate and independent of protein synthesis. Journal of Biological Chemistry. 1992;267(19):13580–13584. [PubMed] [Google Scholar]
- 85.Szalkowski D, White-Carrington S, Berger J, Zhang B. Antidiabetic thiazolidinediones block the inhibitory effect of tumor necrosis factor-α on differentiation, insulin-stimulated glucose uptake, and gene expression in 3T3-L1 cells. Endocrinology. 1995;136(4):1474–1481. doi: 10.1210/endo.136.4.7895657. [DOI] [PubMed] [Google Scholar]
- 86.Zhang B, Berger J, Hu E, et al. Negative regulation of peroxisome proliferator-activated receptor-γ gene expression contributes to the antiadipogenic effects of tumor necrosis factor-α . Molecular Endocrinology. 1996;10(11):1457–1466. doi: 10.1210/mend.10.11.8923470. [DOI] [PubMed] [Google Scholar]
- 87.Ron D, Brasier AR, McGehee RE, Jr., Habener JF. Tumor necrosis factor-induced reversal of adipocytic phenotype of 3T3-L1 cells is preceded by a loss of nuclear CCAAT/enhancer binding protein (C/EBP) Journal of Clinical Investigation. 1992;89(1):223–233. doi: 10.1172/JCI115566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kallen CB, Lazar MA. Antidiabetic thiazolidinediones inhibit leptin (ob) gene expression in 3T3-L1 adipocytes. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5793–5796. doi: 10.1073/pnas.93.12.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hollenberg AN, Susulic VS, Madura JP, et al. Functional antagonism between CCAAT/enhancer binding protein-α and peroxisome proliferator-activated receptor-γ on the leptin promoter. Journal of Biological Chemistry. 1997;272(8):5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- 90.Okuno A, Tamemoto H, Tobe K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. Journal of Clinical Investigation. 1998;101(6):1354–1361. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. Journal of Biological Chemistry. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 92.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends in Endocrinology and Metabolism. 2002;13(2):84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 93.Yu JG, Javorschi S, Hevener AL, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51(10):2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 94.Pajvani UB, Hawkins M, Combs TP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. Journal of Biological Chemistry. 2004;279(13):12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 95.Yamauchi T, Kamon J, Minokoshi Y, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Medicine. 2002;8(11):1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 96.Nawrocki AR, Rajala MW, Tomas E, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. Journal of Biological Chemistry. 2006;281(5):2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 97.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409(6818):307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 98.Banerjee RR, Rangwala SM, Shapiro JS, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303(5661):1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 99.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature. 1997;389(6651):610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 100.Kintscher U, Law RE. PPARγ-mediated insulin sensitization: the importance of fat versus muscle. American Journal of Physiology. 2005;288(2):E287–E291. doi: 10.1152/ajpendo.00440.2004. [DOI] [PubMed] [Google Scholar]
- 101.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ . Annual Review of Biochemistry. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 102.Gavrilova O, Haluzik M, Matsusue K, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. Journal of Biological Chemistry. 2003;278(36):34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 103.Yu S, Matsusue K, Kashireddy P, et al. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. Journal of Biological Chemistry. 2003;278(1):498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- 104.Promrat K, Lutchman G, Uwaifo GI, et al. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39(1):188–196. doi: 10.1002/hep.20012. [DOI] [PubMed] [Google Scholar]
- 105.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled fatty liver improvement with rosiglitazone therapy (FLIRT) trial. Gastroenterology. 2008;135(1):100–110. doi: 10.1053/j.gastro.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 106.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. The New England Journal of Medicine. 2006;355(22):2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 107.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. Journal of Clinical Endocrinology and Metabolism. 2004;89(2):463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 108.Schoonjans K, Peinado-Onsurbe J, Lefebvre A-M, et al. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. The EMBO Journal. 1996;15(19):5336–5348. [PMC free article] [PubMed] [Google Scholar]
- 109.Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. Journal of Biological Chemistry. 1999;274(7):3970–3977. doi: 10.1074/jbc.274.7.3970. [DOI] [PubMed] [Google Scholar]
- 110.Guan H-P, Yong L, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nature Medicine. 2002;8(10):1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 111.Tordjman J, Chauvet G, Quette J, Beale EG, Forest C, Antoine B. Thiazolidinediones block fatty acid release by inducing glyceroneogenesis in fat cells. Journal of Biological Chemistry. 2003;278(21):18785–18790. doi: 10.1074/jbc.M206999200. [DOI] [PubMed] [Google Scholar]
- 112.Buse JB, Tan MH, Prince MJ, Erickson PP. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2004;6(2):133–156. doi: 10.1111/j.1462-8902.2004.00325.x. [DOI] [PubMed] [Google Scholar]
- 113.Miyazaki Y, Mahankali A, Wajcberg E, Bajaj M, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on circulating adipocytokine levels and insulin sensitivity in type 2 diabetic patients. Journal of Clinical Endocrinology and Metabolism. 2004;89(9):4312–4319. doi: 10.1210/jc.2004-0190. [DOI] [PubMed] [Google Scholar]
- 114.Matsui J, Terauchi Y, Kubota N, et al. Pioglitazone reduces islet triglyceride content and restores impaired glucose-stimulated insulin secretion in heterozygous peroxisome proliferator-activated receptor-γ-deficient mice on a high-fat diet. Diabetes. 2004;53(11):2844–2854. doi: 10.2337/diabetes.53.11.2844. [DOI] [PubMed] [Google Scholar]
- 115.Lin C-Y, Gurlo T, Haataja L, Hsueh WA, Butler PC. Activation of peroxisome proliferator-activated receptor-γ by rosiglitazone protects human islet cells against human islet amyloid polypeptide toxicity by a phosphatidylinositol 3′-kinase-dependent pathway. Journal of Clinical Endocrinology and Metabolism. 2005;90(12):6678–6686. doi: 10.1210/jc.2005-0079. [DOI] [PubMed] [Google Scholar]
- 116.Lalloyer F, Vandewalle B, Percevault F, et al. Peroxisome proliferator-activated receptor α improves pancreatic adaptation to insulin resistance in obese mice and reduces lipotoxicity in human islets. Diabetes. 2006;55(6):1605–1613. doi: 10.2337/db06-0016. [DOI] [PubMed] [Google Scholar]