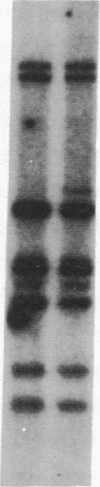
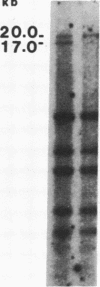
Abstract
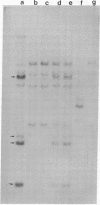
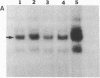
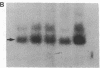
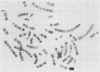
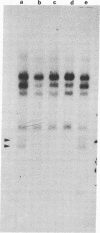
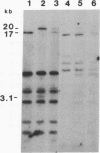
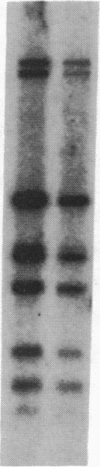
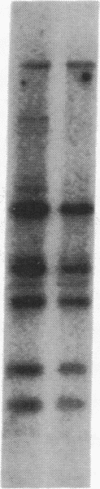
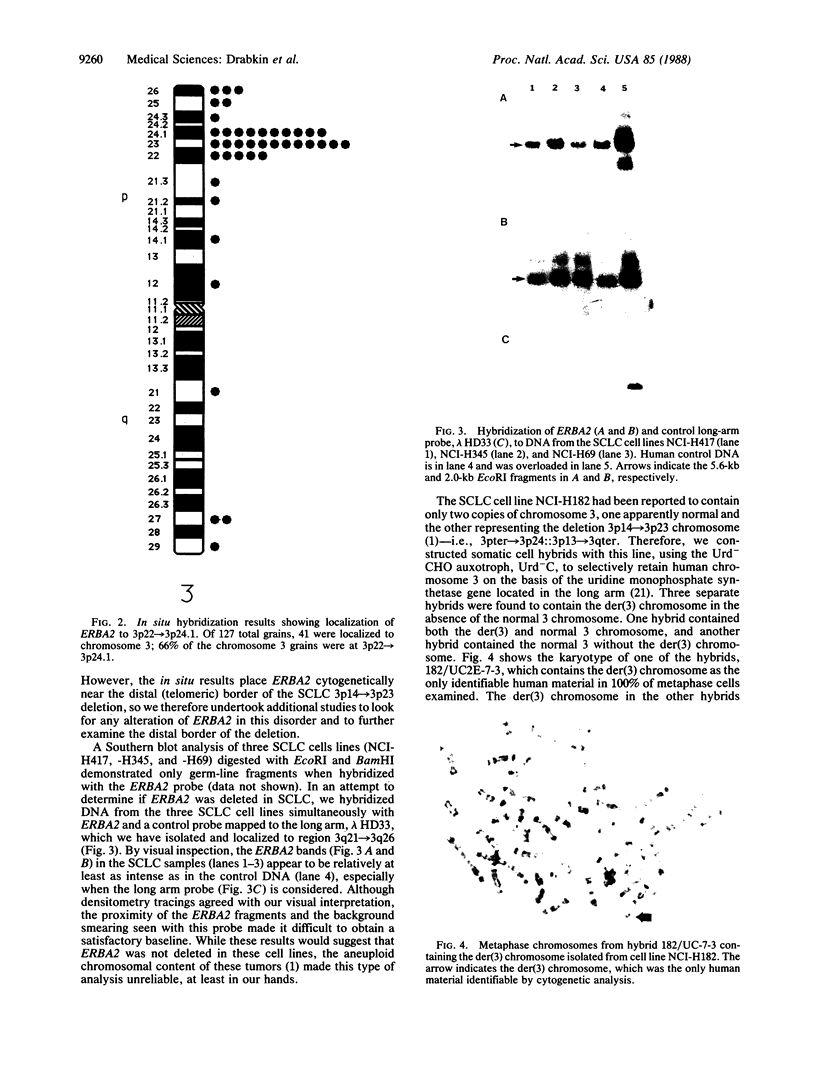
Human genes homologous to the v-erbA oncogene of avian erythroblastosis virus have been mapped to at least two human chromosomes. Recently, the ERBA2 gene was shown to encode a thyroid hormone receptor and localized to chromosome 3 by using flow-sorted chromosomes. We now demonstrate that this gene is located at 3p22----3p24.1, using both somatic cell hybrids and in situ hybridization studies. Since this localization is close to the distal border of the small cell lung cancer (SCLC) 3p14----3p23 deletion, we undertook additional studies to examine the ERBA2 gene in SCLC. Using somatic cell hybrids constructed from the SCLC line NCI-H182 as well as matched patient tumor and control tissue samples, we found that ERBA2 is variably deleted. Therefore, ERBA2 defines at the molecular level the distal border of the SCLC deletion and further implies that the putative suppressor gene is located centromeric of this locus. We also determined that, at least in NCI-H182, the 3p14 breakpoint is proximal to the constitutive 3p14.2 fragile site. These studies would indicate that the mechanism or initiation site of chromosomal rearrangement in SCLC is different from that which occurs during induction of the 3p14 fragile site by aphidicolin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhadeff B., Velivasakis M., Siniscalco M. Simultaneous identification of chromatid replication and of human chromosomes in metaphases of man-mouse somatic cell hybrids. (With 1 color plate). Cytogenet Cell Genet. 1977;19(4):236–239. doi: 10.1159/000130814. [DOI] [PubMed] [Google Scholar]
- Bitter M. A., Neilly M. E., Le Beau M. M., Pearson M. G., Rowley J. D. Rearrangements of chromosome 3 involving bands 3q21 and 3q26 are associated with normal or elevated platelet counts in acute nonlymphocytic leukemia. Blood. 1985 Dec;66(6):1362–1370. [PubMed] [Google Scholar]
- Brauch H., Johnson B., Hovis J., Yano T., Gazdar A., Pettengill O. S., Graziano S., Sorenson G. D., Poiesz B. J., Minna J. Molecular analysis of the short arm of chromosome 3 in small-cell and non-small-cell carcinoma of the lung. N Engl J Med. 1987 Oct 29;317(18):1109–1113. doi: 10.1056/NEJM198710293171803. [DOI] [PubMed] [Google Scholar]
- Carroll P. R., Murty V. V., Reuter V., Jhanwar S., Fair W. R., Whitmore W. F., Chaganti R. S. Abnormalities at chromosome region 3p12-14 characterize clear cell renal carcinoma. Cancer Genet Cytogenet. 1987 Jun;26(2):253–259. doi: 10.1016/0165-4608(87)90059-8. [DOI] [PubMed] [Google Scholar]
- Cohen A. J., Li F. P., Berg S., Marchetto D. J., Tsai S., Jacobs S. C., Brown R. S. Hereditary renal-cell carcinoma associated with a chromosomal translocation. N Engl J Med. 1979 Sep 13;301(11):592–595. doi: 10.1056/NEJM197909133011107. [DOI] [PubMed] [Google Scholar]
- Drabkin H. A., Bradley C., Hart I., Bleskan J., Li F. P., Patterson D. Translocation of c-myc in the hereditary renal cell carcinoma associated with a t(3;8)(p14.2;q24.13) chromosomal translocation. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6980–6984. doi: 10.1073/pnas.82.20.6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gerber M. J., Miller Y. E., Drabkin H. A., Scoggin C. H. Regional assignment of the polymorphic probe D3S3 to 3p14 by molecular hybridization. Cytogenet Cell Genet. 1986;42(1-2):72–74. doi: 10.1159/000132254. [DOI] [PubMed] [Google Scholar]
- Harrison G. S., Drabkin H. A., Kao F. T., Hartz J., Hart I. M., Chu E. H., Wu B. J., Morimoto R. I. Chromosomal location of human genes encoding major heat-shock protein HSP70. Somat Cell Mol Genet. 1987 Mar;13(2):119–130. doi: 10.1007/BF01534692. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Law M. L., Hartz J., Jones C., Zhang X. L., Dewji N., O'Brien J. S., Wenger D. A. Regional localization of the gene coding for sphingolipid activator protein SAP-1 on human chromosome 10. Somat Cell Mol Genet. 1987 Nov;13(6):685–688. doi: 10.1007/BF01534489. [DOI] [PubMed] [Google Scholar]
- Kok K., Osinga J., Carritt B., Davis M. B., van der Hout A. H., van der Veen A. Y., Landsvater R. M., de Leij L. F., Berendsen H. H., Postmus P. E. Deletion of a DNA sequence at the chromosomal region 3p21 in all major types of lung cancer. Nature. 1987 Dec 10;330(6148):578–581. doi: 10.1038/330578a0. [DOI] [PubMed] [Google Scholar]
- LeBeau M. M., Rowley J. D. Heritable fragile sites in cancer. Nature. 1984 Apr 12;308(5960):607–608. doi: 10.1038/308607a0. [DOI] [PubMed] [Google Scholar]
- Markkanen A., Heinonen K., Knuutila S., de la Chapelle A. Methotrexate-induced increase in gap formation in human chromosome band 3p14. Hereditas. 1982;96(2):317–319. doi: 10.1111/j.1601-5223.1982.tb00866.x. [DOI] [PubMed] [Google Scholar]
- Morse H. G., Hays T., Patterson D., Robinson A. Giemsa-11 technique. Applications in the chromosomal characterization of hematologic specimens. Hum Genet. 1982;61(2):141–144. doi: 10.1007/BF00274204. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., Johnson B. E., Minna J. D., Sakaguchi A. Y. Loss of heterozygosity of chromosome 3p markers in small-cell lung cancer. Nature. 1987 Oct 1;329(6138):451–454. doi: 10.1038/329451a0. [DOI] [PubMed] [Google Scholar]
- Patterson D., Jones C., Morse H., Rumsby P., Miller Y., Davis R. Structural gene coding for multifunctional protein carrying orotate phosphoribosyltransferase and OMP decarboxylase activity is located on long arm of human chromosome 3. Somatic Cell Genet. 1983 May;9(3):359–374. doi: 10.1007/BF01539144. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Tommerup N., Nielsen F. A familial reciprocal translocation t(3;7) (p21.1;p13) associated with the Greig polysyndactyly-craniofacial anomalies syndrome. Am J Med Genet. 1983 Nov;16(3):313–321. doi: 10.1002/ajmg.1320160304. [DOI] [PubMed] [Google Scholar]
- Voss R., Lerer I., Povey S., Solomon E., Bobrow M. Confirmation and further regional assignment of aminoacylase 1 (acy-1) on human chromosome 3 using a simplified detection method. Ann Hum Genet. 1980 Jul;44(Pt 1):1–9. doi: 10.1111/j.1469-1809.1980.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Wang N., Perkins K. L. Involvement of band 3p14 in t(3;8) hereditary renal carcinoma. Cancer Genet Cytogenet. 1984 Apr;11(4):479–481. doi: 10.1016/0165-4608(84)90028-1. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Bunn P. A., Jr, Kao-Shan C. S., Lee E. C., Carney D. N., Gazdar A., Minna J. D. A nonrandom chromosomal abnormality, del 3p(14-23), in human small cell lung cancer (SCLC). Cancer Genet Cytogenet. 1982 Jun;6(2):119–134. doi: 10.1016/0165-4608(82)90077-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M. A., Ohyashiki K., Ochi H., Gibas Z., Pontes J. E., Prout G. R., Jr, Huben R., Sandberg A. A. Cytogenetic studies of tumor tissue from patients with nonfamilial renal cell carcinoma. Cancer Res. 1986 Apr;46(4 Pt 2):2139–2147. [PubMed] [Google Scholar]