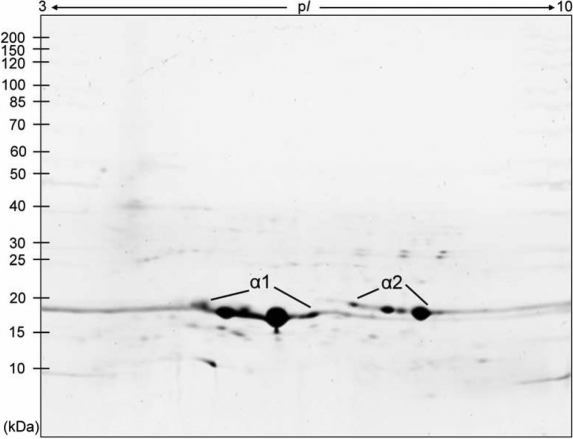
Figure 2.
2-DE gel patterns of αA- and αB-crystallins in a porcine lens. After purification of α-crystallin by gel filtration according to our previous report [5], 100 μg α-crystallin was loaded onto IPG gel strips (pH 3–10 Nonlinear, 13 cm). The IPG strips were rehydrated, and after IEF, subjected to 2-DE. Protein spots marked and enclosed by α1 and α2 on the map denote the acidic αA-crystallin and basic αB-crystallin subunits of native α-crystallin, respectively. They were further digested by trypsin and identified by nano LC-MS/MS. It is noted that only αB-crystallin spots were found to be phosphorylated by gel-based 2-DE proteomic analysis.