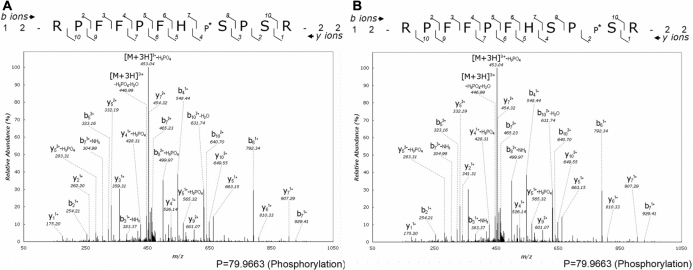
Figure 3.
The representative tandem mass spectra of the phosphorylated peptides 12RPFFPFHS*PSR22 and 12RPFFPFHSPS*R22. A: MS/MS spectrum of the peptide phosphorylated at Ser-19. B: MS/MS spectrum of the peptide phosphorylated at Ser-21. The purified phosphopeptides samples less than 1 μg each from IMAC were first injected into a 2 cm×180 μm capillary trap column followed by LC-MS/MS and spectra collection. Based on the tandem mass spectra of the modified peptides 12RPFFPFHS*PSR22 and 12RPFFPFHSPS*R22 as compared with the original peptide, it can be deduced that either Ser-19 or Ser-21 is phosphorylated. The location of the peptide fragment within the protein is shown by the residue numbers 12 and 22 for the NH2- and COOH-terminus of the phosphorylated peptide sequence. Identified b- and y-ion fragment series are marked by the numbers above and under the peptide sequence, respectively. The putative site of phosphorylation is indicated by * and P* next to serine residues. The mass signals were amplified fivefold, except the ion with the highest intensity.