Figure 7.
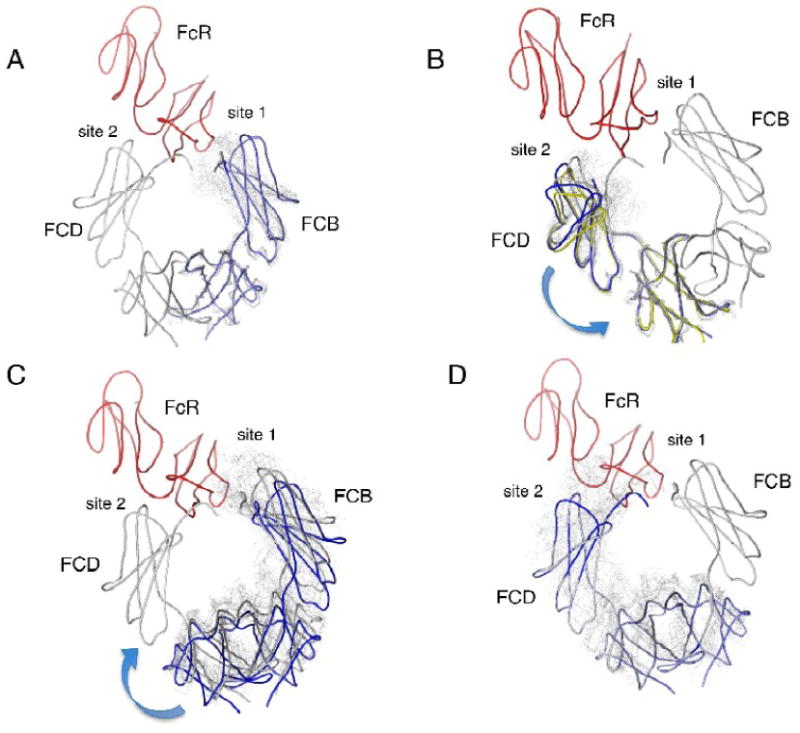
Effects of hinge motions in the IgE-Fc on receptor binding interactions. The structure of the complex between IgE-Fc3-4 and the FcεRIα chain is shown with the IgE-Fc in gray and the α chain in red. The complex Fc subunit FCB will be referred to as chain 1 and the complex Fc subunit FCD as chain 2. Overlaid domains are shown in light gray except as otherwise indicated. Intrachain flexibility is shown in panels (a) and (b) where all chains are overlaid based on their C4 domains and the effect on the subunit's C3 domain is observed. (a) The overlay onto the C4 domain of chain 1. Subunit FCB (chain 1) is shown in light blue. All overlays result in clashes with the α chain. (b) The overlay onto the C4 domain of chain 2 (FCD). All overlays result in clashes with the receptor. Subunits FCB (blue) and O24 (yellow) are more open than FCD and result in few clashes with the receptor, suggesting a possible pathway for dissociation from binding site 2. Interchain flexibility is shown in panels (c) and (d). All chains are overlaid based on their C3 domains, and the resulting new position of the C4 domains is used as the basis for overlaying the rest of the receptor-bound Fc model. The effect on the other C3 domain across the dimer at the other binding site can then be observed. (c) The overlay onto the C3 domain of chain 2 (FCD) at binding site 2. The model shown in blue corresponds to the overlay of FCB, which results in the complete dissociation of chain 1 from site 1. (d) The overlay onto the C3 domain of chain 1 (FCB) at binding site 1. All of the superpositions lead to major steric clashes of chain 2 with site 2.
