Abstract
Primordial germ cells (PGCs) in Xenopus are specified through the inheritance of germ plasm. During gastrulation, PGCs remain totipotent while surrounding cells in the vegetal mass become committed to endoderm through the action of the vegetal localized maternal transcription factor VegT. We find that although PGCs contain maternal VegT RNA, they do not express its downstream targets at the mid-blastula transition (MBT). Transcriptional repression in PGCs correlates with the failure to phosphorylate serine 2 in the carboxy-terminal domain (CTD) of the large subunit of RNA polymerase II (RNAPII). As serine 5 is phosphorylated, these results are consistent with a block after the initiation step but before the elongation step of RNAPII-based transcription. Repression of PGC gene expression occurs despite an apparently permissive chromatin environment. Phosphorylation of CTD-serine 2 and expression of zygotic mRNAs in PGCs are first detected at neurula, some 10 hours after MBT, indicating that transcription is significantly delayed in the germ cell lineage. Significantly, Oct-91, a POU subclass V transcription factor related to mammalian Oct3/4, is among the earliest zygotic transcripts detected in PGCs and is a likely mediator of pluripotency. Our findings suggest that PGCs are unable to respond to maternally inherited endoderm determinants because RNAPII activity is transiently blocked while these determinants are present. Our results in a vertebrate system further support the concept that one strategy used repeatedly during evolution for preserving the germline is RNAPII repression.
Keywords: Xenopus, Oogenesis, Germline determination, Germ plasm, Transcriptional repression, Pol II
INTRODUCTION
Specification of both the germline and somatic cell lineages is established quite early in Xenopus through the inheritance of specific maternal RNAs that localize to the vegetal pole during oogenesis. Germline RNAs and proteins are found in germ plasm, a cytoplasmic domain exclusive to germ cells that becomes deposited within the oocyte vegetal cortex. In the early embryo, germ plasm passes asymmetrically to one daughter cell of mitotic pairs, yielding a small number of germ plasm bearing blastomeres. Only cells receiving sufficient amounts of germ plasm will remain totipotent and give rise to primordial germ cells (PGCs), the future gametes of the organism (reviewed by Houston and King, 2000). The other blastomeres are fated to become endoderm through the action of the maternal transcription factor VegT, which is also localized to the vegetal cortex (Zhang et al., 1998; Casey et al., 1999; Xanthos et al., 2001). Commitment to an endoderm fate occurs by early gastrula stage, as shown in single cell transplantation assays (Wylie et al., 1987). Thus, both the future germline and the endodermal lineage originate from a common vegetal cytoplasm. An important question is, how does the presence of germ plasm promote totipotency and prevent an endodermal fate in PGCs?
In Drosophila and Caenorhabditis elegans, global repression of mRNA transcription is one mechanism that prevents the germ cell lineage from responding to regional signals specifying somatic fates (Seydoux et al., 1996; Van Doren et al., 1998; Strome and Lehmann, 2007; Timinszky et al., 2008). In the germline of both these invertebrate model systems, RNA polymerase II (RNAPII) activity is temporarily blocked through regulating the phosphorylated state of the heptapeptide repeat YSPTSPS (>40 tandem copies) found in the carboxy-terminal domain (CTD). During a normal round of transcription, unphosphorylated RNAPII is recruited to promoters where, within the initiation complex, each CTD-serine 5 is phosphorylated (P-Ser5) by the cyclin-dependent kinase CDK7 (for reviews, see Bensaude et al., 1999; Price, 2000; Phatnani and Greenleaf, 2006). Initiation is followed by an elongation step that requires the phosphorylation of serine 2 (P-Ser2) by the cyclin-dependent kinase CDK9. Thus, specific serine phosphorylation events have been correlated with transcriptional initiation and elongation events. Recycling of RNAPII for additional rounds of transcription requires the action of CTD-phosphatases, another potential point of regulation (Cho et al., 1999). In Drosophila and C. elegans, specific monoclonal antibodies directed at P-Ser5 and P-Ser2 revealed that expression is very low or missing in the germline, in sharp contrast to the neighboring somatic cells (Seydoux and Dunn, 1997; Deshpande et al., 1999; Deshpande et al., 2005; Ghosh and Seydoux, 2008).
Germline transcriptional repression in C. elegans requires OMA-1/OMA-2 very early (Guven-Ozkan et al., 2008) and PIE-1 later (Seydoux et al., 1996). Recent evidence indicates that OMA-1/OMA-2 retains TAF-4 (TATA-binding protein associated factor 4) in the cytoplasm (Guven-Ozkan et al., 2008) and that PIE-1 completely inhibits CDK7 and partially blocks CDK9 activity, which suggests that both initiation and elongation steps are targets for repression. Interestingly, however, it is the repression of CDK7 that is essential for transcriptional repression and germ line specification in vivo (Ghosh and Seydoux, 2008). In Drosophila, polar granule component (pgc) is essential for repressing CTD-Ser2 phosphorylation, and in pgc-null mutants pole cells eventually degenerate before reaching the gonad (Hanyu-Nakamura et al., 2008; Timinszky et al., 2008). Polar Granule Component was shown to directly interact with CDK9 but not CDK7, and to somehow prevent CDK9 from being recruited to transcription sites (Hanyu-Nakamura et al., 2008). Neither PIE-1 nor Polar Granule Component have homologs in any other species, suggesting that although general mechanisms are conserved between these two systems, the effector molecules may not be (reviewed by Strome and Lehmann, 2007).
Transcriptional repression in germ line precursor cells has also been observed in the ascidian Halocynthia roretzi, a simple chordate (Tomioka et al., 2002). The mammalian germline might also use global transcriptional repression as a strategy to protect PGC identity during migration, as has been recently documented in the mouse (Seki et al., 2007). Here, both P-Ser5 and P-Ser2 are missing. In Xenopus, no similar studies have been attempted, although Dziadek and Dixon did examine RNA synthesis in endoderm and PGCs with [3H]-uridine and found no differences (Dziadek and Dixon, 1977).
Several laboratories have examined the relationship between transcriptional repression and histone modifications in promoting the preservation of the germline. In C. elegans, PIE-1 activity is replaced by histone H3 methyltransferase complexes that shift the chromatin to a more condensed state (Schaner et al., 2003; Wang et al., 2005). As soon as they are formed, Drosophila pole cells have high levels of a histone H3 conserved modification (H3meK9) found in silenced genomic regions. Thus, flies have both modes of repression during the same developmental time period. During PGC migration in the mouse, the germline undergoes remodeling with a loss of repressive chromatin, but prevents inappropriate gene expression by repressing RNAPII, as previously mentioned (Seki et al., 2007).
In Xenopus, Palancade et al. have shown distinct cycles of RNAPII phosphorylation that also correlate with active transcription (Palancade et al., 2001). At fertilization, the CTD-RNAPII is almost completely de-phosphorylated with only minor amounts of the phosphorylated form present, as determined using the same monoclonal antibodies (H5 and H14) that were employed in the earlier studies (Patturajan et al., 1998; Seydoux and Dunn, 1997; Palancade et al., 2001; Ghosh and Seydoux, 2008). Consistent with these observations, transcription is reduced to very low levels. At the initiation of strong zygotic transcription, twelve divisions later at MBT, CTD-RNAPII becomes hyperphosphorylated and P-Ser2 and P-Ser5 attain maximum levels of expression.
To determine whether global transcriptional repression might be functioning in the Xenopus germline, we isolated pure populations of PGCs at pre- and post-MBT stages and examined the phosphorylated state of CTD-Ser2 and CTD-Ser5. We find that although the endoderm determinant VegT RNA is present in PGCs, its immediate downstream targets are not expressed at MBT. We show that whereas somatic cells gain a hyperphosphorylated form of RNAPII at the MBT, such phosphorylation events are delayed by ten hours in PGCs until neurula. Consistent with that finding, suppression subtractive hybridization (SSH) also did not detect new transcripts in PGCs until neural stages. Significant changes in chromatin remodeling that could account for global transcriptional repression were not detected. However, differences in histone linker protein and DNA methylation were detected that are consistent with preserving an undifferentiated state in PGCs during these early stages. The mechanism of PGC transcriptional repression appears to target the CTD of the RNAPII enzyme, as has been found in the C. elegans and the Drosophila germline. Activation of the RNAPII by hyperphosphorylation of the CTD appears to be a pivotal regulatory event on which evolution has converged to maintain the germline in both invertebrates and vertebrates.
MATERIALS AND METHODS
Xenopus embryos and isolation of PGCs
Adult frogs were purchased from Xenopus One or Xenopus Express. Ovulated eggs from human chorionic gonadotropin (hCG)-induced females were fertilized in vitro for embryo production and embryos staged according to the normal table of Nieuwkoop and Faber (Nieuwkoop and Faber, 1956).
PGCs were isolated at embryonic stages 8, 10 and 14 as described by Venkataraman et al. (Venkataraman et al., 2004), but using a higher dose of DiOC6(3). Briefly, stage 2 embryos were dejellied and stained at the four-cell stage with DiOC6(3) (Molecular Probes). A stock solution (2:1000, 0.1×MMR buffer) was made from a saturated solution of DiOC6 in DMSO. Embryos were stained in the dark for twenty minutes with a 4:1000 dilution of the stock, rinsed thoroughly, and kept in the dark until the appropriate stage. At the desired stage, embryos were dissociated in a Ca2+-, Mg2+-free medium [all buffers have been previously described by Sive et al. (Sive et al., 2000)], the animal cap removed, and the PGCs within the endodermal mass manually selected for further analysis (Fig. 1).
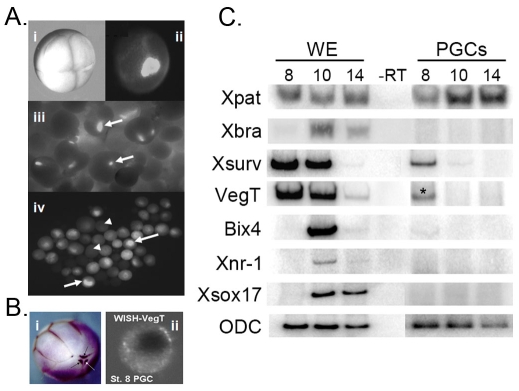
Fig. 1.
PGC isolation and gene expression analyses in Xenopus embryos. (A) PGCs can be identified based on the distinctive fluorescence staining of the germ plasm with DiOC6. (i) Eight-cell embryo and (ii) corresponding image after stereofluorescence microscopy showing DiOC6 staining of vegetal pole germ plasm. (iii) PGCs near the floor of the blastocoel just after removal of the animal cap (arrows). (iv) Stereofluorescence microscopy of PGCs (arrows) and unstained somatic cells (arrowheads) from embryos dissociated at gastrula (stage 10). (B) VegT RNA is found in PGCs. WISH with anti-VegT probe of (i) early embryo and (ii) isolated PGC from a blastula-stage embryo. (C) Zygotic genes are not expressed in PGCs at MBT. Gene expression in PGCs isolated before (stage 8) and after (stages 10 and 14) MBT was compared with that in whole embryos (WE) by semi-quantitative RT-PCR with gene-specific primers. Xpat, PGC-specific marker; Xbra, mesoderm marker; Bix4, Xnr1 and Xsox17, endodermal-specific and downstream targets of maternal VegT; Xsurv (survivin), a maternal gene. Presence of Xpat but not Xbra or zygotic VegT at stage 10 and 14 indicates that PGCs were not contaminated with mesoderm. Zygotic VegT, a spliced variant of maternal VegT, is expressed at post-MBT stages in the mesoderm. Ornithine decarboxylase (ODC) served as a control. PGCs did not express endoderm markers (Bix4, Xnr1, Xsox17), but did contain maternal VegT RNA (asterisk).
Immunofluorescence (IF) and confocal imaging
For Figs 2, 3 and Fig. 5A, dissociated cells were fixed in MEMFA for one hour at room temperature [MEMFA: 5×MEM buffer, 10 mM MOPS (pH 7.0), 1 mM sodium acetate, 0.5 mM EDTA (pH 8.0), 10.0 mM EGTA, 5.0 mM MgSO4, final pH 7.5]. MEMFA fixative is freshly made from two parts 5×MEM buffer, one part 37% formaldehyde (Sigma) and seven parts H2O. Cells were blocked with 500 μl of PBT and 10% goat serum at 4°C for 1 hour. Cells were incubated with primary antibodies in fresh blocking solution overnight at 4°C then with appropriate fluorescent secondary antibodies. For confocal analysis, images were acquired on an inverted Zeiss LSM-510 Confocal Laser Scanning Microscope equipped with Argon ion, Helium-Neon and Green-Neon lasers.
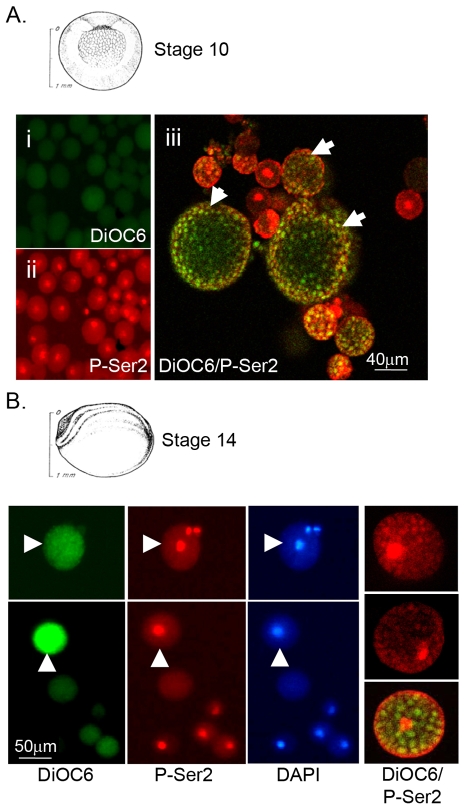
Fig. 2.
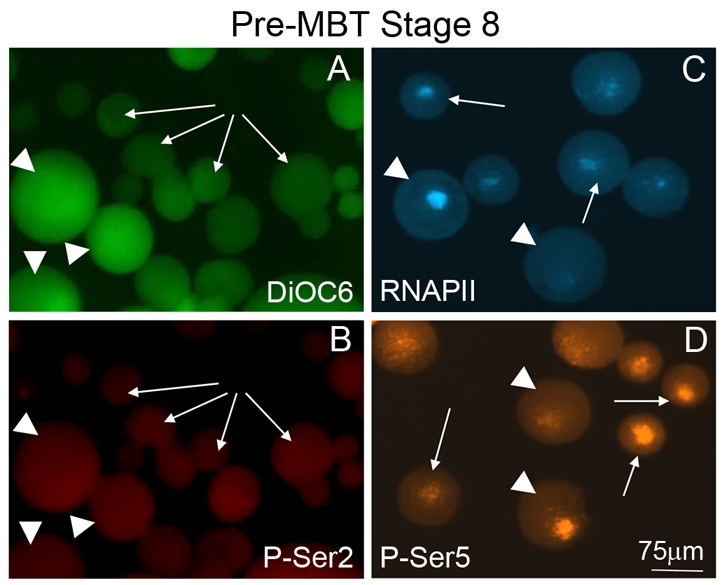
Pre-MBT PGCs and somatic cells express CTD-P-Ser5, but not CTD-P-Ser2. Immunofluorescence of dissociated cells isolated from pre-MBT stage 8 embryos previously stained with DiOC6 (A) to identify PGCs. Monoclonal antibodies H5 and H14 were used to detect the expression of CTD phospho-serines 2 (B, P-Ser2) and 5 (D, P-Ser5), respectively, in PGCs (arrowheads) and somatic cells (arrows). Monoclonal antibody 8WG16 recognizes the unphosphorylated RNA Pol II large subunit (C, RNAPII) and served as a positive control.
Fig. 3.
PGC expression of CTD-P-Ser2 is delayed until neurula stage. The phosphorylation status of the CTD RNA Pol II in PGCs and somatic cells at two post-MBT stages, gastrulation (A, stage 10) and neurula (B, stage 14). (A) Dissociated somatic cells show very low DiOC6 staining (i) and strong nuclear P-Ser2 reactivity (red; ii). (iii) Merged confocal image of P-Ser2 (red) and DiOC6 (green) staining of dissociated somatic cells and PGCs. The P-Ser2 epitope is detected in somatic cells, but not PGCs (arrowheads). (B) Dissociated stage 14 somatic cells and PGCs (arrowheads) labeled with DiOC6 and stained for P-Ser2. Nuclei were stained with DAPI; note the somatic cell without a nucleus. The last panel shows a merged confocal image of P-Ser2 (red) and DiOC6 (green) staining of isolated somatic cells and PGCs. Strong nuclear P-Ser2 staining is now evident in PGCs.
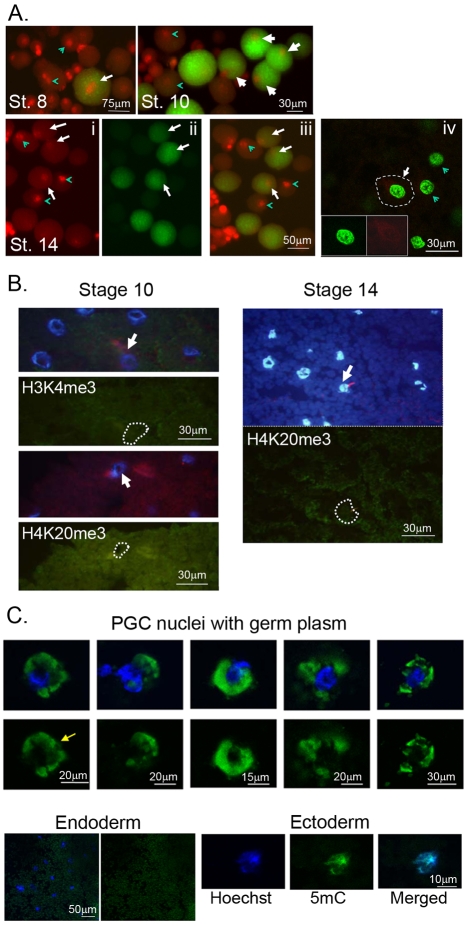
Fig. 5.
Pre-neurula PGCs display permissive chromatin and histone staining. (A) Fluorescence stereomicroscopy of PGCs (green) and somatic cells from dissociated embryos. Cells were stained with anti-hyperacetylated Histone H4 (Penta, red), a marker of transcriptionally active chromatin. Stage 8 and 10 represent merged images of Penta immunostaining and DiOC6 labeling (green). Stage 14: (i) Penta alone, (ii) DiOC6, (iii) merged image, (iv) merged confocal image showing Penta staining (green) and a PGC (outlined) identified by Xnos1 (red) immunostaining. (insets show separate images). Examples of PGCs (white arrows) and endoderm cells (green arrowheads) with nuclear staining. Both PGCs and somatic cells express Histone H4 Penta at every stage. (B) Cryostat sections from stages 10 and 14 showing the endodermal region immunostained for H4K20me3 or H3K4me3 (green). PGCs indicated by Xnos1 immunostaining (red); nuclei stained with Hoechst (blue). PGCs (white arrows), other nuclei from endoderm cells. PGC nuclei are outlined. No nuclear staining was detected for any of the histone methylated lysines tested (see Materials and methods). (C) Stage 10 isolated PGC and endoderm nuclei (blue) immunostained for 5mC (green). Both cell types were demethylated on cytosine residues. DiOC6-stained germ plasm remained associated with PGC nuclei (arrow). 5mC staining of ectoderm nuclei served as a positive control.
All other samples were fixed in Dent's solution (80% methanol/20% DMSO) overnight at –20°C. For IF of cryostat sections, fixed samples were embedded in OCT and sectioned at 12 microns. Sections were extracted in PBS containing 0.25% Triton X-100 and blocked in 5% heat-inactivated donkey serum/1% BSA in PBS containing 0.05% Triton X-100 (PBT). The sections were incubated with primary antibody overnight at 4°C, rinsed in PBT, and incubated at room temperature for one hour with respective secondary antibodies. After a rinse in PBS, sections were mounted in Fluorogel in TES buffer (Electron Microscopy Sciences).
For detection of 5-methylcytosine (5mC), Xenopus nuclei were isolated from stage 10 dissociated embryos and treated following the procedures of Stancheva et al. (Stancheva et al., 2002). Samples were incubated with anti-5mC primary antibody for 1 hour at room temperature, and then with Alexa 488 secondary antibody for 30 minutes.
Primary and secondary antibodies
Primary antibodies were: monoclonal mouse antibody (Research Diagnostic, catalog number RDI-RNAPOLII-H5) diluted at 1:100; H14 (Covance, catalog number MMS-134R), 1:100; G16 (Covance, catalog number MS-126R), 1:200; anti-dimethyl-histone H3 (Lys4; Upstate, catalog number 07-030), 1:300; anti-hyperacetylated Histone H4 (Penta; Upstate, catalog number 06-946), 1:1000; and anti-dimethyl-histone H3 (Lys9; Upstate catalog number 07-212), 1:250.
H4K20me3 (Abcam, catalog number ab9053), 1:500; H3K4me3 (Abcam, catalog number ab1012), 1:100; H3K27me3 (Cell Signaling, catalog number 9756), 1:400; H3K27me2 (Cell Signaling, catalog number 97555), 1:500; 5-methylcytosine (Abcam, catalog number ab10805), 1: 200; affinity purified anti-Xnos1 antibody, made in goats against total recombinant expressed Xnos1 protein (Invitrogen), 1:50 or 1:100. Secondary antibodies were as follows: Alexa 350 (Invitrogen, catalog number A10035), 1:1000; Alexa 488 (Invitrogen, catalog number A21206), 1:500; Alexa 555 (Molecular Probes, catalog number A21432), 1:500; and Cy3 anti-mouse antibody (Jackson ImmunoResearch, catalog number 115-165-146), used at a dilution of 1:500. To visualize nuclei, DAPI (Sigma, 1 mg/ml) was used at a dilution of 1:3000, or Hoechst (Invitrogen, H3570, 10 mg/ml) was used at a dilution of 1:10,000 for 15 minutes at room temperature. Cells were treated with the SlowFade Antifade from Molecular Probes (catalog number S-2828) and mounted for microscopic observations.
Whole-mount in situ hybridization
Whole-mount in situ hybridization (WISH) and histological procedures were performed as detailed by Houston et al. (Houston et al., 1998). VegT antisense digoxigenin-labeled RNA probes were generated by linearizing the B12 clone with NotI and subsequent transcription with SP6 RNA polymerase (Zhang and King, 1996).
Analysis of gene expression using RT-PCR
Total RNA was isolated from 50-100 PGCs at the various stages by using Stratagene's Absolutely RNA microprep kit and following the detailed instructions provided. Semi-quantitative RT-PCR analysis of gene expression in PGCs was as described by Venkataraman et al. (Venkataraman et al., 2004). The primers used are referenced and the annealing temperatures were as noted: Xpat at 56°C (Hudson and Woodland, 1998); Xbra at 56°C (Wilson and Melton, 1994); VegT at 55°C (Zhang and King, 1996); Xnr1 (Lustig et al., 1996); Bix-4 at 62°C and ODC at 55°C (Xanthos et al., 2001); Xsox17 at 55°C (Hudson et al., 1997); Xsurv (BE191778) at 62°C (forward: TAAAACCTCAGCACACTTCCAAC; reverse: TTCCTTTCCGTGACAAAGTGTGTC); Oct-25 (F: TAATGG AGAGATGCTTGATG; R: TTCTCTATGTTCGTCCTCC); Oct-60 (F: CCATATTGTACAGCCAAACCTC; R: GTTCCAGTCAAAGG AA GCAG); Oct-91 (F: CAGATGGCAGCGGACAG; R: CAACT GG TTGGCAGAATCC) (Dunican et al., 2008); B4 (F: GGCT GAAGTTTTTGCTACGG; R: CTTTTGGGTTTCCTGCTCTG); H1c (F: CCTCAAGTTGGCTCTCAAGG; R: CCTTCTTGGGCTTTTTAGGG); Xdnmt1 (F: TTGGCTTCCTTGAAGCAGAT; R: AGGTACCATTG GTGGAGCAG). Amplified products were analyzed on 5% polyacrylamide/1×TBE gels and exposed to PhosphorImager screens (Molecular Dynamics).
Suppression subtractive hybridization
cDNA subtraction was performed using Clontech's PCR-Select cDNA Subtraction Kit. The procedures were modified from O'Neill and Sinclair (O'Neill and Sinclair, 1997), as detailed by Venkataraman et al. (Venkataraman et al., 2004).
Virtual northern
Transcripts identified by SSH were used as probes to verify their expression pattern. Full-length PGC cDNA from stages 8, 10 and 14 were resolved on a 1% agarose gel and transferred to a nylon membrane. Resulting blots were probed with clones of candidate genes in pDrive vector (Qiagen), restricted with EcoR1 and radiolabeled by random prime labeling (Amersham DNA Mega prime kit).
RESULTS
Primordial germ cells contain both germline and somatic cell determinants
The vegetal cortex is a common site for a diverse population of maternally localized RNAs that determine both germline and somatic cell fates. Previous results from whole-mount in situ hybridization (WISH) suggest that blastomeres arising from the vegetal pole could inherit both germ plasm RNAs, as well as other localized RNAs, such as the endoderm determinate, maternal VegT (mVegT). To determine if PGCs contain determinants for both somatic and germline fates, we asked whether VegT could be detected in PGCs by WISH, as well as by RT-PCR analysis of isolated PGCs.
To isolate PGCs, we took advantage of the very high content of mitochondria in germ plasm and employed the lipophilic fluorescent dye DiOC6 to selectively stain mitochondria in living embryos. Using a low concentration of the dye, PGCs were easily distinguished from somatic cells in the vegetal region of the embryo (Fig. 1A). PGCs of DiOC6-stained embryos developed in a similar fashion to the untreated control embryos. The correct number of PGCs was produced and their migration into the dorsal mesentery was normal, indicating that the DiOC6 had no adverse affects (data not shown) (Venkataraman et al., 2004). Fluorescent PGCs were manually selected from embryos dissociated at stage 8 that had been raised in the dark to protect against phototoxicity. In situ hybridization, as well as the RT-PCR results, indicated that indeed maternal VegT RNA was present, perhaps at a lower level, in PGCs of the eight-cell and blastula embryos (Fig. 1B,C; see VegT* lane). VegT is also expressed zygotically (zVegT) as a splice variant of maternal VegT, but only in the mesoderm. Therefore, an important question is: what prevents PGCs from developing as endoderm?
Zygotic genes are not expressed in PGCs at MBT
One explanation for the failure of the transcription factor VegT to specify endoderm fate in PGCs is that these cells are transcriptionally repressed at the mid-blastula transition (MBT) whereas somatic cells are not. Such a phenomenon has been described in both the C. elegans and the Drosophila germlines (Seydoux and Fire, 1994; Seydoux et al., 1996; Van Doren et al., 1998), and might be true in ascidians as well (Tomioka et al., 2002). The downstream targets of the transcription factor mVegT have been identified and include the transcription factors Bix4 and Xsox17 and the growth factor Xnr1 (Hudson et al., 1997; Casey et al., 1999; Takahashi et al., 2000; Zhang et al., 2005). Bix4 and Xsox17 are required for endoderm specification and Xnr1 is capable of inducing mesoderm in the marginal zone. We investigated whether these downstream targets of mVegT were expressed in PGCs that were isolated before the mid-blastula transition (pre-MBT; stage 8), well after zygotic transcription had been initiated (post-MBT; stage 10), or at early neurula (stage 14). PGCs were isolated at these stages and gene expression analyzed by RT-PCR using specific primers for the corresponding transcripts. As expected, the isolated PGCs expressed the PGC-specific marker Xpat, but not the mesoderm marker Xbra, indicating that our selection of PGCs was accurate. However, although PGCs contained mVegT and the household enzyme ornithine decarboxylase (ODC), they did not express zygotic VegT (zVegT), or the targets of mVegT: Bix4, Xsox17 and Xnr1. As expected, zVegT, Bix4, Xnr1 and Xsox17 were all strongly expressed after MBT and declined by neurula as indicated by the sibling-matched whole embryo controls (Fig. 1C). Maternally expressed survivin (Xsurv), a positive regulator of cell cycle progression localized to the mitotic spindle (Murphy et al., 2002; Yang et al., 2004), appears prematurely downregulated in PGCs when compared with whole embryos, possibly reflecting differences in cell cycle regulation (Whitington and Dixon, 1975). From these results, we conclude that PGCs do not express endodermal genes at MBT, in contrast to their endodermal neighbors. Our results suggest that PGCs might be transcriptionally repressed at MBT.
Transcriptional elongation but not initiation is blocked in PGCs
In C. elegans and Drosophila, a transient global repression of mRNA transcription protects the germ cell lineage from responding to cues initiating somatic cell differentiation. In both of these organisms, transcriptional repression is correlated with the failure to phosphorylate serine 2 within the CTD of RNAPII, as previously mentioned (Seydoux et al., 1996; Van Doren et al., 1998; Strome and Lehmann, 2007; Timinszky et al., 2008). CTD-Ser2 phosphorylation is required for transcriptional elongation events (Wada et al., 1998; Yamaguchi et al., 1999), whereas CTD-Ser5 phosphorylation is associated with initiation events (Pei et al., 2001; Phatnani and Greenleaf, 2006). To test for the presence of phosphorylated serines in the RNAPII CTD in isolated PGCs or somatic cells, we used the monoclonal antibodies H5 and H14 to detect P-Ser2 and P-Ser5, respectively. H5 and H14 specifically detect these serine phospho-epitopes in a wide range of eukaryotes including Drosophila, C. elegans and Xenopus (Bregman el al., 1995; Kim et al., 1997; Seydoux and Dunn, 1997; Palancade et al., 2001). As expected, the CTD-P-Ser2 epitope was not detected in pre-MBT embryos (Fig. 2, stage 8), but was detected strongly in somatic cells following the MBT (Fig. 3A; stage 10). In sharp contrast, PGCs remained negative for CTD-P-Ser2 well after the MBT (Fig. 3Aiii, arrowheads). Therefore, the absence of zygotic transcripts in PGCs correlated with the absence of the P-Ser2 phospho-epitope. Interestingly, at all stages tested, nuclei in both somatic cells and PGCs were strongly positive for nuclear RNAPII and the P-Ser5 epitope (Fig. 2C,D). These results indicated that initiation complexes were present in both PGCs and somatic cells prior to MBT. Our findings strongly suggest a model in which RNAPII function in PGCs is blocked at MBT at a step prior to elongation but after initiation.
Zygotic transcription initiates at Neurula in PGCs
If PGCs are transcriptionally silenced to prevent them from responding to maternal and zygotic somatic cell determinants, then one might expect that PGCs would initiate their own transcriptional program at the conclusion of these developmental events. In Xenopus, maternal transcripts have declined and early patterning is essentially completed by neurula stages. Indeed, we found that PGCs first express the CTD-P-Ser2 epitope at the neural plate stage, some 10 hours after MBT (Fig. 3B, stage 14). These results indicate that PGCs might be significantly delayed in initiating their genetic program, well past the time when somatic cells become transcriptionally active.
To look more directly at transcription in PGCs and to identify new zygotic transcripts, we turned to a PCR-based subtractive cloning strategy (suppression subtractive hybridization; SSH) (Ji et al., 2002). RNA was extracted from isolated PGCs at stages 8, 10 and 14. SSH yields amplified products that are different between two populations of RNAs. If PGCs are transcriptionally silent at the MBT but active at neurula as the IF data suggest, then SSH between PGC transcripts isolated at stages 8 and 10 should not yield any amplified products, whereas SSH between stages 10 and 14 should. The efficiency of subtraction was verified by analyzing the levels of Xnos1 [formerly known as Xcat2 (Mosquera et al., 1993)], a PGC-specific Nanos family member, in the subtracted and unsubtracted cDNA (data not shown). As expected, unsubtracted samples (Fig. 4A, lane U) appeared as a heterogeneously sized smear of cDNA products. Amplified products were first detected between gastrulation and the neural plate stage. These data, together with the IF analyses, strongly support our conclusion that PGCs are transcriptionally quiescent until neurula (Fig. 4A, stage 10-stage 14).
Fig. 4.
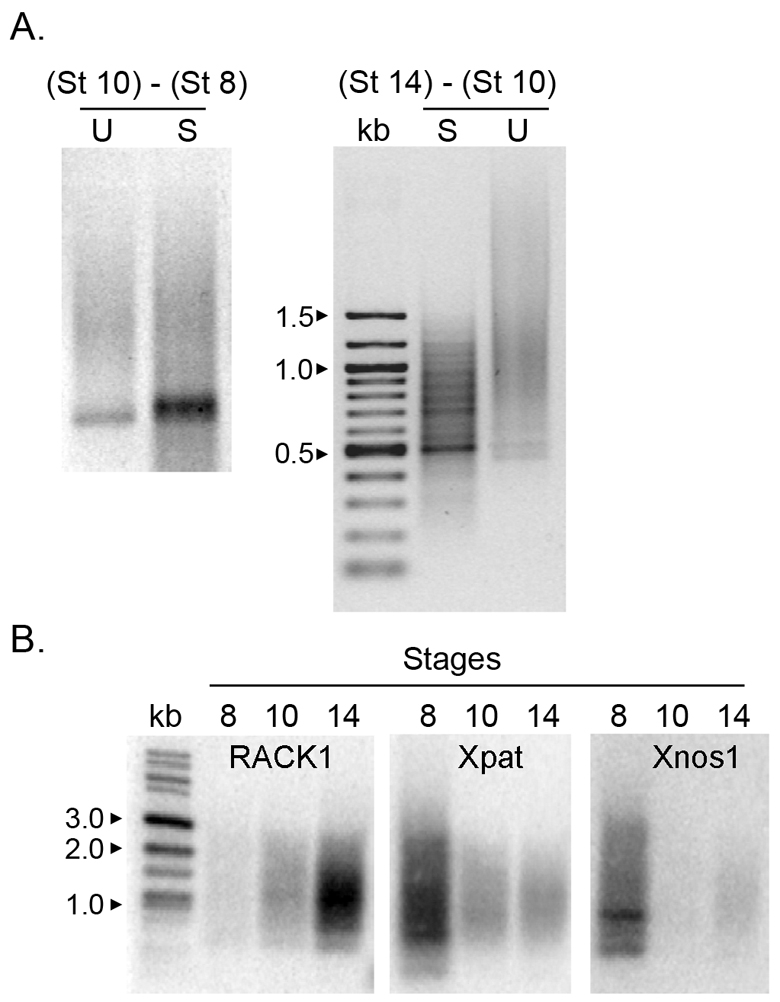
PGCs are transcriptionally active at neurula stage. (A) New PGC transcripts are detected at neurula stage. Suppression subtractive hybridization (SSH, see Materials and methods) was used to identify transcripts newly expressed in PGCs. Transcripts from stage 8 PGCs were subtracted from those isolated from stage 10 PGCs [(stage 10) – (stage 8)]. The amplification step failed to detect any novel transcripts made between these two stages, consistent with PGCs being transcriptionally silent during this time. Novel transcripts were detected in PGCs as discrete amplified bands after the subtraction of stage 10 PGC transcripts from stage 14 [(stage 14) – (stage 10)]. U, unsubtracted/amplified (control); S, subtracted/amplified (experimental). (B) Rack1 is a PGC zygotic transcript. Bands were excised from the gel shown in A in three size ranges (0.5-0.7 kb, 0.7-1.0 kb and 1.0-1.5 kb) and cloned. Randomly selected clones were used as probes against a blot of stage 8, 10 and 14 PGC cDNAs and zygotically expressed clones selected. Rack1 was identified by sequencing. Maternal Xpat and Xnos1 RNAs served as controls. Kb, size marker.
Identification of PGC zygotic transcripts: RACK1 and Oct-91
PGC mRNAs transcribed at neurula might be expected to encode proteins required for continued PGC development and survival. To identify what genes might be represented in the SSH-amplified products, bands were excised from the gel and cloned. Seven clones that gave a strong hybridization signal when probed with PGC cDNA were tested for their temporal expression; one was confirmed to be zygotically expressed at neurula by virtual northern. This clone was 99% (497/501) identical in sequence to Xenopus Receptor for Activated C-Kinase 1 (RACK1; GenBank accession number AF105259) (Kwon et al., 2001) (Fig. 4B). RACK1 belongs to a family of proteins involved in anchoring activated PKC to appropriate cellular organelles. As discussed below, Oct-91 was also identified as a PGC transcript at neurula (see Fig. 6C). Neither RACK1 nor Oct-91 are germline specific but are also expressed in somatic cells. However, these results show at a minimum that RACK1 and Oct-91 increased significantly in neurula stage PGCs and thus they provide additional evidence that the presence of P-Ser2 in PGCs coincides with transcriptional activity.
Fig. 6.
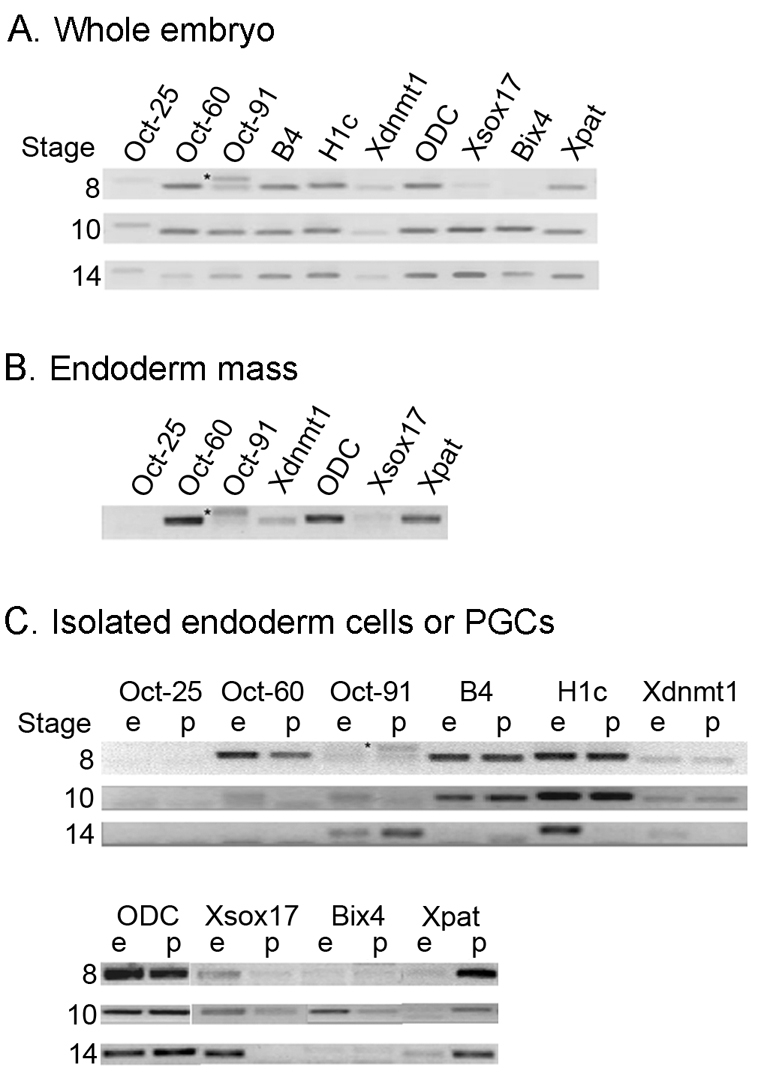
PGCs activate Oct-91 and repress H1c linker histone expression at neurula stage. Gene expression before and after MBT analyzed by semi-quantitative RT-PCR. (A) Whole embryo controls. (B) Gene expression analyzed in isolated endoderm masses (pre-MBT stages 7.5-8). (C) Gene expression in PGCs (p) and endoderm (e) isolated pre-MBT (stage 8) and post-MBT (stages 10, 14). Oct-25 and Oct-60, maternal genes; Oct-91, zygotic gene; histone linker proteins maternal B4 and H1c; Xdnmt1, Xenopus DNA methyltransferase; ornithine decarboxylase (ODC) served as a control; endoderm zygotic markers Xsox17 and Bix4; Xpat, a PGC-specific marker. The presence of Xpat but not Bix4 or Xsox17 at stages 10 and 14 indicates that PGCs were not contaminated during isolation. Asterisk indicates a nonspecific amplification product for Oct-91.
Chromatin structure in PGCs at MBT favors transcription
In addition to regulating RNAP II activity, global silencing of mRNA transcription could be achieved at the level of chromatin structure. To address this point in a general way, we sought to determine whether there was an obvious difference in the complement of histone modifications, expression of histone linkers, or levels of 5-methyl cytosine (5mC) between somatic cells and PGCs around the time of the MBT. Di-methylated lysine 4 of histone H3 (H3meK4) or hyperacetylated histone H4 (Penta) are markers of transcriptionally active chromatin. Inactive chromatin is marked by methylation of H3 lysine 9, 4 or 27 (H3K9, H3K4me3, H3K27me2, H3K27me3) and histone H4 lysine 20me3 (Schaner et al., 2003; Yin and Lin, 2007; Shilatifard, 2008; Dunican et al., 2008; Katz et al., 2009). No significant differences were detected in staining between the somatic cells and the PGCs at any stage (Fig. 5). All cells stained for markers of active but not inactive chromatin (Fig. 5A,B). HeLa cells served as a positive control for antibody staining (not shown).
Xenopus oocytes and early cleavage stages, unlike the mouse, retain high levels of methylated DNA (5mC) that progressively decline reaching the lowest levels at the MBT (Stancheva et al., 2002). We investigated whether PGC nuclei retained 5mC content through the extended period of transcriptional repression. Isolated nuclei from PGCs and endoderm at stage 10 were compared for 5mC by immunofluorescence microscopy (Fig. 5C). No differences in staining were detected between the two lineages, indicating that PGCs and endoderm had undergone demethylation of 5mC (Fig. 5C). By contrast, 5mC was detected in ectoderm. Finally, we analyzed the expression of maternal DNA methyltransferase (xDnmt1) and two linker histones in pre-and post-MBT endoderm and PGCs (Fig. 6). No differences in xDnmt1 RNA expression were detected between whole embryos, endoderm or PGCs at the different stages. Consistent with this observation, Stancheva and Meehan (Stancheva and Meehan, 2000) did not detect any xDnmt1 protein in the vegetal pole of early-cleaved embryos, indicating that, at most, xDnmt1 plays a minor role in PGC or endoderm transcriptional repression. A different story emerged from the analysis of RNAs for the histone linker proteins B4 and H1c. By neurula stages, oocyte-specific B4 linker protein is completely replaced by H1 variants shown to prevent chromatin remodeling in somatic cells (Saeki et al., 2005). As expected, B4 and H1c RNA were detected in endoderm and PGCs through gastrulation, with B4 RNA being undetectable by neurula (Fig. 6C). Importantly, although H1c RNA continues to be expressed at neurula in the now specified endoderm, H1c is not expressed in PGCs as they initiate their genetic program. We conclude that transcriptional repression in Xenopus PGCs does not involve a dramatic alteration of chromatin architecture and occurs in spite of a `permissive' chromatin environment.
Zygotic expression of Oct-91 in PGCs
The maintenance of an undifferentiated state is preserved in mouse PGCs and embryonic stem cells in part through the POU domain transcription factor Oct3/4. Xenopus Oct-60, Oct-25 and Oct-91 are in the same POU subclass as Oct3/4 (Hinkley et al., 1992). Oct3/4 behaves as a functional homolog for Oct-60 or Oct-25 in rescue type experiments (Cao et al., 2006). Oct-60 and Oct-25 are expressed maternally and predominantly in the animal pole region. Recently, Oct-25 and Oct-60 have been shown to repress the target genes of maternal VegT and β-catenin, consistent with a role in maintaining pluripotency (Cao et al., 2007; Cao et al., 2008). We sought to determine what the status of these Oct genes was in Xenopus PGCs (Fig. 6). Interestingly, we found that Oct-25 was expressed at low levels in the whole embryo (Fig. 6A), but was not detected at any stage in the endoderm or in PGCs (Fig. 6B,C). In contrast to previous work (Whitfield et al., 1993), we did detect Oct-60 in the endoderm and in PGCs at stage 8, but expression was undetectable by the end of gastrulation. Thus, neither maternal Oct-60 nor Oct-25 was present in PGCs post-MBT at a time when PGCs were transcriptionally repressed. By contrast, Oct-91 is zygotically expressed; expression peaks at gastrulation and declines by stage 14 in whole embryos (Hinkley et al., 1992) (see Fig. 6A; stage 8 consistently showed a non-specific smear or two bands). Oct-91 was weakly expressed in the endoderm at stages 10 and 14 but not expressed in PGCs at stage 10, consistent with our earlier data showing that PGCs were transcriptionally repressed at this stage. Importantly, Oct-91 was expressed in PGCs at stage 14. De novo PGC Oct-91 expression provides additional evidence that PGCs are transcriptionally active at neurula. Most importantly, these results identify a transcription factor that is capable of promoting pluripotency in the germ cell lineage, and suggest that Oct-91 is the functional equivalent of mouse Oct3/4 in PGCs.
DISCUSSION
Global repression of RNAPII transcription in PGCs
Nothing intrinsically prevents PGCs from becoming somatic cells, underscoring the crucial role repression plays in maintaining PGC identity (Seydoux et al., 1996; Hayashi et al., 2004). Several lines of evidence presented in this work lead to the conclusion that global transcriptional repression of RNAPII operates in Xenopus to preserve the germline. First, a required step for transcriptional elongation at MBT, the phosphorylation of CTD-Ser2, fails to occur in PGCs. Second, despite the presence of the VegT transcription factor, endoderm gene expression is not initiated in PGCs. Third, mRNA synthesis is detected in the germline only after the primary germ layers are specified. Coincidentally, the expression of a transcription factor associated with pluripotency, Oct-91, is initiated.
Dziadek and Dixon had previously shown that Xenopus PGCs and their endodermal neighbors are identical in their ability to incorporate [3H]-uridine into RNA (Dziadek and Dixon, 1977). However, these studies could not distinguish the activities of RNAPI, II or III. In light of our findings that RNAPII is inactive, these earlier observations can now be interpreted as indicating that ribosomal RNA synthesis is not affected in the germline, but resumes at MBT, as it does in somatic cells. Similar observations were made in C. elegans and Drosophila (Seydoux and Dunn, 1997). Thus, a specific mechanism operates in these germlines to repress RNAPII but not RNAPI/III activity. Interestingly, both pre-MBT PGCs and somatic cells contain CTD-P-Ser5, suggesting that initiation events have occurred and that all cells of the Xenopus blastula are primed for transcription. Palancade et al. also detected P-Ser5 but not P-Ser2 in pre-MBT embryos (Palancade et al., 2001). Our data do not rule out the possibility that Ser5 is less phosphorylated in PGCs and that this somehow contributes to blocking transcription. For example, in C. elegans reduced levels of P-Ser5 are essential for the preservation of germ cell fate, and PIE-1 interacts with both kinases, CDK9 and CDK7, to reduce phosphorylation (Ghosh and Seydoux, 2008).
Transient RNAPII repression might be quite common in germlines. In the mouse where PGCs form after gastrulation and do not require germ plasm, migrating PGCs also lack P-Ser5 and P-Ser2 (Seki et al., 2007). Ascidians and zebrafish offer less clear but potentially instructive examples. In Halocynthia roretzi, germline precursor cells are transcriptionally repressed even in the presence of CTD-P-Ser2; however, P-Ser5 was not examined leaving the question open (Tomioka et al., 2002). Knaut et al. ruled out transcriptional repression in the zebrafish germline (Knaut et al., 2000); however, they did report that vasa expression in PGCs is delayed one hour past the initiation time of general somatic transcription. Again, the status of CTD-Ser5 and whether other transcripts besides vasa were affected remains to be investigated. The transient loss of RNAPII activity in the germline could operate directly by inhibiting the kinase that phosphorylates P-Ser2 (P-TEFb: cyclinT/CDK9), or indirectly through a phosphatase activity or perhaps by preventing association of the kinase with its substrate; all potential mechanisms to test.
New genetic program expressed in PGCs
Subtractive cloning (SSH) confirmed that novel zygotic genes were not transcribed in PGCs until neurula stages, coincident with the appearance of P-Ser2. Surprisingly, few clones were recovered by SSH and none represented PGC-specific genes. Because SSH would select only for newly expressed genes, these results might indicate that the initial gene program in PGCs is largely a zygotic re-expression of maternal genes. Alternatively, these RNAs might simply be of low abundance and/or SSH is not an effective means for selecting them. Rack1 expression was confirmed by blotting, but could not be detected by WISH, which further suggests that early PGC transcripts might be present at low copy number.
The PGC genetic program would be expected to accomplish two goals: preserve the germline by continuing to prevent endoderm or other somatic fates, and promote migration to the gonads. The two genes identified as new PGC transcripts are consistent with these goals. RACK1 has been described as a scaffolding protein capable of integrating different signaling pathways (Nilsson et al., 2004). For example, RACK1 bound to the IP3 receptor regulates intracellular calcium levels, and thus could be involved in regulating migration and adhesion events in PGCs (Sklan et al., 2006). The most intriguing finding, however, was that Oct-91, a POU-V protein related to mouse Oct3/4 pluripotency factor, is expressed in PGCs at the same time that its expression is waning in committed somatic cells. In mice, Oct3/4 is required for the maintenance of PGCs and ES cell self-renewal (Kehler et al., 2004; Niwa et al., 2000). Importantly, Xenopus Oct-91 can maintain self-renewal in Oct-4-deficient ES cells and does so more effectively than either Oct-60 or Oct-25 (Morrison and Brickman, 2006). Conversely, Xenopus embryos deficient in Oct-91 prematurely express differentiation genes and the resulting abnormal phenotype can be rescued by mouse Oct-4 expression. Our findings rule out Oct-25 and Oct-60 as players in maintaining Xenopus PGC pluripotency, as neither one is present at the correct time or place for this function (Cao et al., 2007; Cao et al., 2008). Surprisingly, Xenopus embryos deficient in all three Oct genes apparently exhibit normal germ cell development, thus leaving open the question of what role Oct-91 might play in PGCs (Morrison and Brickman, 2006).
PGC chromatin structure is permissive not repressive
Nascent mouse PGCs display a characteristic `signature' of inactive chromatin that includes H3K27me3, H3K4me3 and 5mC (Hajkova et al., 2008). Drosophila germ cells lag behind somatic cells in expressing active chromatin (Schaner et al., 2003). By contrast, we found that both PGCs and somatic cells display robust staining for histones characteristic of active chromatin regardless of their transcriptional state. At the same time, none of the six histone marks for inactive chromatin or 5mC were detected, although H3K4me3 and H4K20me3 were detected in whole embryos by neurula (Dunican et al., 2008). Unlike the mouse, there is no global demethylation or imprinting in Xenopus. Any such modifications are likely to exist in a subset of inactive genes and thus were not detectable by our histological approach.
Our data are consistent with transcriptional silencing being extended in PGCs some 10 hours past MBT. xDnmt functions to preserve gene inactivity in somatic cells prior to the MBT by direct action as a transcription repressor, not as a methyltransferase (Dunican et al., 2008). No differences in xDnmt1 RNA expression were noted in PGCs or endoderm, and xDnmt1 seems to account for specific rather than global gene repression. Interestingly, xDnmt1 represses Oct-91 and Xsox17 pre-MBT. Future studies will determine how repression of Oct-91 is released at neurula while Xsox17 remains repressed in PGCs. Another component in chromatin repression occurs at the level of histone linker proteins. The replacement of oocyte B4 with the more restrictive H1c variant is complete by neurula and is required for specific gene repression and normal somatic cell development (Steinbach et al., 1997; Smith et al., 1988; Saeki et al., 2005). PGCs contain maternal H1c RNA, but this is completely lost by neurula when gene transcription initiates. Thus, a linker protein that restricts chromatin remodeling is repressed in PGCs but expressed in somatic cells. It will be important to discover what histone linker variant replaces B4 in PGCs.
Model: molecular sequestration of the germline
Our findings are most consistent with a model (Fig. 7) in which PGCs fail to activate transcription at the MBT, and thus cannot respond to the maternal molecular cues for endoderm specification. By neurula (stage 14), both the maternal determinant VegT and its downstream targets are no longer detected (Fig. 1), and the opportunity to induce endoderm fates in PGCs is missed. We propose that sequestration through the repression of gene expression while maintaining a permissive chromatin structure accounts for the preservation of an uncommitted state in Xenopus PGCs. By presenting this model, we do not preclude other mechanisms that might be working in concert to preserve PGC totipotency, including RNA degradation and translational repression. On the contrary, mechanisms in addition to transcriptional repression are likely to be operating in PGCs to ensure maternal determinants like VegT do not function.
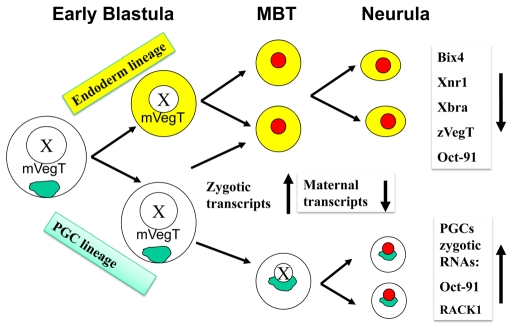
Fig. 7.
Model for PGC molecular sequestration. During the early cleavage stages, germ plasm (green) segregates asymmetrically into a few vegetal pole cells, the future PGCs. Daughter cells lacking germ plasm (yellow) are fated to become endoderm. Both PGCs and endoderm cells contain the maternal endoderm determinant VegT. At MBT, somatic cells become transcriptionally active (red nuclei) and transcribe the zygotic downstream targets of VegT, Bix4 and Xnr1, thus setting the endoderm fate. PGCs are not transcriptionally active (white nucleus) and cannot respond to VegT. Maternal transcripts, including VegT, decline dramatically by neurula, at which time PGCs initiate their own program of transcription that includes Oct-91 and Rack1. We propose that transcription is delayed in PGCs to prevent them from entering an endodermal fate.
Germ plasm must contain the components required to cause global transcriptional repression in PGCs and we are currently searching for these components. Neither PIE-1 nor Polar Granule Component is found outside their taxon, yet other germline repressors, such as Nanos, are conserved from hydra to humans (Torras and González-Crespo, 2005). It will be important to determine whether Xenopus Nanos1 plays a similar role repressing somatic gene expression (Hayashi et al., 2004; Tsuda et al., 2003; Wang et al., 2005; Köprunner et al., 2001; Asaoka et al., 1998; Asaoka-Taguchi et al., 1999; Deshpande et al., 1999; Deshpande et al., 2005). Regardless of the mediators, our findings further support molecular sequestration through transcriptional repression as a mechanism used repeatedly during evolution for preserving PGC totipotency.
Acknowledgments
The authors acknowledge the outstanding technical assistance of Elio Dancausse; the encouragement and advice from Drs G. Seydoux and R. Lehmann, and the services rendered by the DRI Imaging Center at UMSM. We thank Michael Danilchik for the WISH shown in Fig. 1 and Hugh Woodland for the Xpat and Xsox17 plasmids used in this study. This work was supported by NIH grant GM33932 to M.L.K. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
References
- Asaoka M., Sano H., Obara Y., Kobayashi S. (1998). Maternal Nanos regulates zygotic gene expression in germline progenitors of Drosophila melanogaster. Mech. Dev. 78, 153-158 [DOI] [PubMed] [Google Scholar]
- Asaoka-Taguchi M., Yamada M., Nakamura A., Hanyu K., Kobayashi S. (1999). Maternal Pumilio acts together with Nanos in germline development in Drosophila embryos. Nat. Cell Biol. 1, 431-437 [DOI] [PubMed] [Google Scholar]
- Bensaude O., Bonnet F., Cassé C., Dubois M. F., Nguyen V. T., Palancade B. (1999). Regulated phosphorylation of the RNA polymerase II C-terminal domain (CTD). Biochem. Cell Biol. 77, 249-255 [PubMed] [Google Scholar]
- Bregman D. B., Du L., van der Zee S., Warren S. L. (1995). Transcription dependent redistribution of the large subunit of RNA polymerase II to discrete nuclear domains. J. Cell Biol. 129, 287-298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Siegel D., Knochel W. (2006). Xenopus POU factors of subclass V inhibit activin/nodal signaling during gastrulation. Mech. Dev. 123, 614-625 [DOI] [PubMed] [Google Scholar]
- Cao Y., Siegel D., Donow C., Knochel S., Yuan L., Knochel W. (2007). POU-V factors antagonize maternal Veg T activity and β-Catenin signaling in Xenopus embryos. EMBO J. 26, 2942-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Siegel D., Oswald F., Knöchel W. (2008). Oct25 represses transcription of nodal/activin target genes by interaction with signal transducers during Xenopus gastrulation. J. Biol. Chem. 283, 34168-34177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey E. S., Tada M., Fairclough L., Wylie C. L., Heasman J., Smith J. C. (1999). Bix4 is activated directly by VegT and mediates endoderm formation in Xenopus development. Development 126, 4193-4200 [DOI] [PubMed] [Google Scholar]
- Cho H., Kim T. K., Mancebo H., Lane W. S., Flores O., Reinberg D. (1999). A protein phosphatase functions to recycle RNA polymerase II. Genes Dev. 13, 1540-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G., Calhoun G., Yanowitz J. L., Schedl P. D. (1999). Novel functions of nanos in down-regulating mitosis and transcription during the development of the Drosophila germline. Cell 99, 271-281 [DOI] [PubMed] [Google Scholar]
- Deshpande G., Calhoun G., Jinks T. M., Polydorides A. D., Schedl P. (2005). Nanos down-regulates transcription and modulates CTD phosphorylation in the soma of early Drosophila embryos. Mech. Dev. 122, 645-657 [DOI] [PubMed] [Google Scholar]
- Dunican D. S., Ruzov A., Hackett J. A., Meehan R. R. (2008). xDnmt1 regulates transcriptional silencing in pre-MBT Xenopus embryos independently of its catalytic function. Development 135, 1295-1302 [DOI] [PubMed] [Google Scholar]
- Dziadek M., Dixon K. E. (1977). An autoradiographic analysis of nucleic acid synthesis in the presumptive primordial germ cells of Xenopus laevis. J. Embryol. Exp. Morphol. 37, 13-31 [PubMed] [Google Scholar]
- Ghosh D., Seydoux G. (2008). Inhibition of transcription by the Caenorhabditis elegans germline protein PIE-1: genetic evidence for distinct mechanisms targeting initiation and elongation. Genetics 178, 235-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven-Ozkan T., Nishi Y., Robertson S. M., Lin R. (2008). Global transcriptional repression in C. elegans germline precursors by regulated sequestration of TAF-4. Cell 135, 149-160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajkova P., Ancelin K., Waldmann T., Lacoste N., Lange U. C., Cesari F., Lee C., Almouzni G., Schneider R., Surani M. A. (2008). Chromatin dynamics during epigenetic reprogramming in the mouse germ line. Nature 452, 877-881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyu-Nakamura K., Sonobe-Nojima H., Tanigawa A., Lasko P., Nakamura A. (2008). Drosophila Pgc protein inhibits P-TEFb recruitment to chromatin in primordial germ cells. Nature 451, 730-733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Hayashi M., Kobayashi S. (2004). Nanos suppresses somatic cell fate in Drosophila germ line. Proc. Natl. Acad. Sci. USA 101, 10338-10342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkley C. S., Martin J. F., Leibham D., Perry M. (1992). Sequential expression of multiple POU proteins during amphibian early development. Mol. Cell. Biol. 12, 638-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston D. W., King M. L. (2000). Germ plasm and molecular determinants of germ cell fate. Curr. Top. Dev. Biol. 50, 155-181 [DOI] [PubMed] [Google Scholar]
- Houston D. W., Zhang J., Maines J. Z., Wasserman S. A., King M. L. (1998). A Xenopus DAZ-like gene encodes an RNA component of germ plasm and is a functional homologue of Drosophila boule. Development 125, 171-180 [DOI] [PubMed] [Google Scholar]
- Hudson C., Woodland R. (1998). Xpat, a gene expressed specifically in germ plasm and primordial germ cells of Xenopus laevis. Mech. Dev. 73, 159-168 [DOI] [PubMed] [Google Scholar]
- Hudson C., Clements D., Friday R. V., Stott D., Woodland H. R. (1997). Xsox17 alpha and beta mediate endoderm formation in Xenopus. Cell 91, 397-405 [DOI] [PubMed] [Google Scholar]
- Ji W., Wright M. B., Cai L., Flament A., Lindpaintner K. (2002). Efficacy of SSH PCR in isolating differentially expressed genes. BMC Genomics 3, 12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. J., Edwards T. M., Reinke V., Kelly W. G. (2009). A C. elegans LSD1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell 137, 308-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehler J., Tolkunova E., Koschorz B., Pesce M., Gentile L., Boiani M., Lomeli H., Nagy A., McLaughlin K. J., Scholer H. R., et al. (2004). Oct4 is required for primordial germ cell survival. EMBO Rep. 5, 1078-1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E., Du L., Bregman D. B., Warren S. L. (1997). Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA. J. Cell Biol. 136, 19-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaut H., Pelegri F., Bohmann K., Schwarz H., Nüsslein-Volhard C. (2000). Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 149, 875-888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köprunner M., Thisse C., Thisse B., Raz E. (2001). A zebrafish nanos-related gene is essential for the development of primordial germ cells. Genes Dev. 15, 2877-2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H. J., Bae S., Son Y. H., Chung H. M. (2001).Expression of the Xenopus homologue of the receptor for activated C-kinase 1 (RACK1) in the Xenopus embryo. Dev. Genes Evol. 211, 195-197 [DOI] [PubMed] [Google Scholar]
- Lustig K. D., Kroll K., Sun E. E., Kirschner M. W. (1996). Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodernal patterning and blastopore lip formation. Development 122, 4001-4012 [DOI] [PubMed] [Google Scholar]
- Morrison G. M., Brickman J. M. (2006). Conserved roles for Oct4 homologues in maintaining multipotency during early vertebrate development. Development 133, 2011-2022 [DOI] [PubMed] [Google Scholar]
- Mosquera L., Forristall C., Zhou Y., King M. L. (1993). A mRNA localized to the vegetal cortex of Xenopus oocytes encodes a protein with a nanos-like zinc finger domain. Development 117, 377-386 [DOI] [PubMed] [Google Scholar]
- Murphy C. R., Sabel J. L., Sandler A. D., Dagle J. M. (2002). Survivin mRNA is down-regulated during early Xenopus laevis embryogenesis. Dev. Dyn. 225, 597-601 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1956). Normal Table of Xenopus laevis (Daudin), 1st edn. Amsterdam: North-Holland Publishing Company; [Google Scholar]
- Nilsson J., Sengupta J., Frank J., Nissen P. (2004). Regulation of eukaryotic translation by the RACK1 protein: a platform for signaling molecules on the ribosome. EMBO Rep. 5, 1137-1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H., Miyazaki J., Smith A. G. (2000). Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 24, 372-376 [DOI] [PubMed] [Google Scholar]
- O'Neill M. J., Sinclair A. H. (1997). Isolation of rare transcripts by representational difference analysis. Nucleic Acids Res. 25, 2681-2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palancade B., Bellier S., Almouzni G., Bensaude O. (2001). Incomplete RNA polymerase II phosphorylation in Xenopus laevis early embryos. J. Cell Sci. 114, 2483-2489 [DOI] [PubMed] [Google Scholar]
- Patturajan M., Schulte R. J., Sefton B. M., Berezney R., Vincent M., Bensaude O., Warren S. L., Corden J. L. (1998). Growth-related changes in phosphorylation of yeast RNA polymerase II. J. Biol. Chem. 273, 4689-4694 [DOI] [PubMed] [Google Scholar]
- Pei Y., Hausmann S., Ho C. K., Schwer B., Shuman S. (2001). The length, phosphorylation state, and primary structure of the RNA polymerase II carboxyl-terminal domain dictate interactions with mRNA capping enzymes. J. Biol. Chem. 276, 28075-28082 [DOI] [PubMed] [Google Scholar]
- Phatnani H. P., Greenleaf A. L. (2006). Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20, 2922-2936 [DOI] [PubMed] [Google Scholar]
- Price D. H. (2000). P-TEFb, a cyclin-dependent kinase controlling elongation by RNA polymerase II. Mol. Cell. Biol. 20, 2629-2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki H., Ohsumi K., Aihara H., Ito T., Hirose S., Ura K., Kaneda Y. (2005). Linker histone variants control chromatin dynamics during early embryogenesis. Proc. Natl. Acad. Sci. USA 102, 5697-5702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaner C. E., Deshpande G., Schedl P., Kelly W. G. (2003). A conserved chromatin architecture marks and maintains the restricted germ cell lineage in worms and flies. Dev. Cell 5, 747-757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Y., Yamaji M., Yabuta Y., Sano M., Shigeta M., Matsui Y., Saga Y., Tachibana M., Shinkai Y., Saitou M. (2007). Cellular dynamics associated with the genome-wide epigenetic reprogramming in migrating primordial germ cells in mice. Development 134, 2627-2638 [DOI] [PubMed] [Google Scholar]
- Seydoux G., Fire A. (1994). Soma-germline asymmetry in the distributions of embryonic RNAs in Caenorhabditis elegans. Development 120, 2823-2834 [DOI] [PubMed] [Google Scholar]
- Seydoux G., Dunn M. A. (1997). Transcriptionally repressed germ cells lack a subpopulation of phosphorylated RNA polymerase II in early embryos of Caenorhabditis elegans and Drosophila melanogaster. Development 124, 2191-2201 [DOI] [PubMed] [Google Scholar]
- Seydoux G., Mello C. C., Pettitt J., Wood W. B., Priess J. R., Fire A. (1996). Repression of gene expression in the embryonic germ lineage of C. elegans. Nature 382, 713-716 [DOI] [PubMed] [Google Scholar]
- Shilatifard A. (2008). Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr. Opin. Cell Biol. 20, 341-348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sive H., Grainger R., Harland R. (2000). Early Development of Xenopus laevis: A Laboratory Manual Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Sklan E. H., Podoly E., Soreq H. (2006). RACK1 has the nerve to act: structure meets function in the nervous system. Prog. Neurobiol. 78, 117-134 [DOI] [PubMed] [Google Scholar]
- Smith R. C., Dworkin-Rastl E., Dworkin M. B. (1988). Expression of a histone H1-like protein is restricted to early Xenopus development. Genes Dev. 2, 1284-1295 [DOI] [PubMed] [Google Scholar]
- Stancheva I., Meehan R. R. (2000). Transient depletion of xDnmt1 leads to premature gene activation in Xenopus embryos. Genes Dev. 14, 313-327 [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Stancheva I., El-Maarri O., Walter J., Niveleau A., Meehan R. R. (2002). DNA methylation at promoter regions regulates the timing of gene activation in Xenopus laevis embryos. Dev. Biol. 243, 155-165 [DOI] [PubMed] [Google Scholar]
- Steinbach O. C., Wolffe A. P., Rupp R. A. (1997). Somatic linker histones cause loss of mesodermal competence in Xenopus. Nature 389, 395-399 [DOI] [PubMed] [Google Scholar]
- Strome S., Lehmann R. (2007). Germ versus soma decisions, lessons from flies and worms. Science 316, 392-393 [DOI] [PubMed] [Google Scholar]
- Takahashi S., Yokota C., Takano K., Tanegashima K., Onuma Y., Goto J., Asashima M. (2000). Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127, 5319-5329 [DOI] [PubMed] [Google Scholar]
- Timinszky G., Bortfeld M., Ladurner A. G. (2008). Repression of RNA polymerase II transcription by a Drosophila oligopeptide. PLoS ONE 3, e2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M., Miya T., Nishida H. (2002). Repression of zygotic gene expression in the putative germline cells in ascidian embryos. Zool. Sci. 19, 49-55 [DOI] [PubMed] [Google Scholar]
- Torras R., González-Crespo S. (2005). Posterior expression of nanos orthologs during embryonic and larval development of the anthozoan Nematostella vectensis. Int. J. Dev. Biol. 49, 895-899 [DOI] [PubMed] [Google Scholar]
- Tsuda M., Sasaoka Y., Kiso M., Abe K., Haraguchi S., Kobayashi S., Saga Y. (2003). Conserved role of nanos proteins in germ cell development. Science 301, 1239-1241 [DOI] [PubMed] [Google Scholar]
- Van Doren M., Williamson A. L., Lehmann R. (1998). Regulation of zygotic gene transcription in Drosophila primordial germ cells. Curr. Biol. 8, 243-246 [DOI] [PubMed] [Google Scholar]
- Venkataraman T., Dancausse E., King M. L. (2004). PCR-based Cloning and Differential Screening of RNAs from Xenopus Primordial Germ Cells, Cloning Uniquely Expressed RNAs from Rare Cells. Methods in Molecular Biology: Germ Cell Protocols (Molecular Embryo Analysis, Live Imaging, Transgenesis and Cloning), Vol.2 (ed. H. Schatten), 67-78 Totowa: Humana Press; [DOI] [PubMed] [Google Scholar]
- Wada T., Takagi T., Yamaguchi Y., Watanabe D., Handa H. (1998). Evidence that P-TEFb alleviates the negative effect of DSIF on RNA polymerase II-dependent transcription in vitro. EMBO J. 17, 7395-7403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Kennedy S., Conte D., Jr, Kim J. K., Gabel H. W., Kamath R. S., Mello C. C., Ruvkun G. (2005). Somatic misexpression of germline P granules and enhanced RNA interference in retinoblastoma pathway mutants. Nature 436, 593-597 [DOI] [PubMed] [Google Scholar]
- Whitfield T., Heasman J., Wylie C. (1993). XLPOU-60, a Xenopus POU-domain mRNA, is oocyte-specific from very early stages of oogenesis, and localized to presumptive mesoderm and ectoderm in the blastula. Dev. Biol. 155, 361-370 [DOI] [PubMed] [Google Scholar]
- Whitington P. M., Dixon K. E. (1975). Quantitative studies of germ plasm and germ cells during early embryogenesis of Xenopus laevis. J. Embryol. Exp. Morphol. 33, 57-74 [PubMed] [Google Scholar]
- Wilson P., Melton D. (1994). Mesodermal patterning by an inducer gradient depends on secondary cell-cell communication. Curr. Biol. 4, 676-686 [DOI] [PubMed] [Google Scholar]
- Wylie C., Snape A., Heasman J., Smith J. C. (1987). Vegetal pole cells and commitment to form endoderm in Xenopus laevis. Dev. Biol. 119, 496-502 [DOI] [PubMed] [Google Scholar]
- Xanthos J. B., Kofron M., Wylie C., Heasman J. (2001). Maternal VegT is the initiator of a molecular network specifying endoderm in Xenopus laevis. Development 128, 167-180 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Takagi T., Wada T., Yano K., Furuya A., Sugimoto S., Hasegawa J., Handa H. (1999). NELF, a multisubunit complex containing RD, cooperates with DSIF to repress RNA polymerase II elongation. Cell 97, 41-51 [DOI] [PubMed] [Google Scholar]
- Yang D., Welm A., Bishop J. M. (2004). Cell division and cell survival in the absence of survivin. Proc. Natl. Acad. Sci. USA 101, 15100-15105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., Lin H. (2007). An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature 450, 304-308 [DOI] [PubMed] [Google Scholar]
- Zhang C., Basta T., Fawcett S. R., Klymkowsky M. W. (2005). SOX7 is an immediate-early target of VegT and regulates Nodal-related gene expression in Xenopus. Dev. Biol. 278, 526-541 [DOI] [PubMed] [Google Scholar]
- Zhang J., King M. L. (1996). Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesoderm patterning. Development 122, 4119-4129 [DOI] [PubMed] [Google Scholar]
- Zhang J., Houston D. W., King M. L., Payne C., Wylie C., Heasman J. (1998). The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell 94, 515-524 [DOI] [PubMed] [Google Scholar]