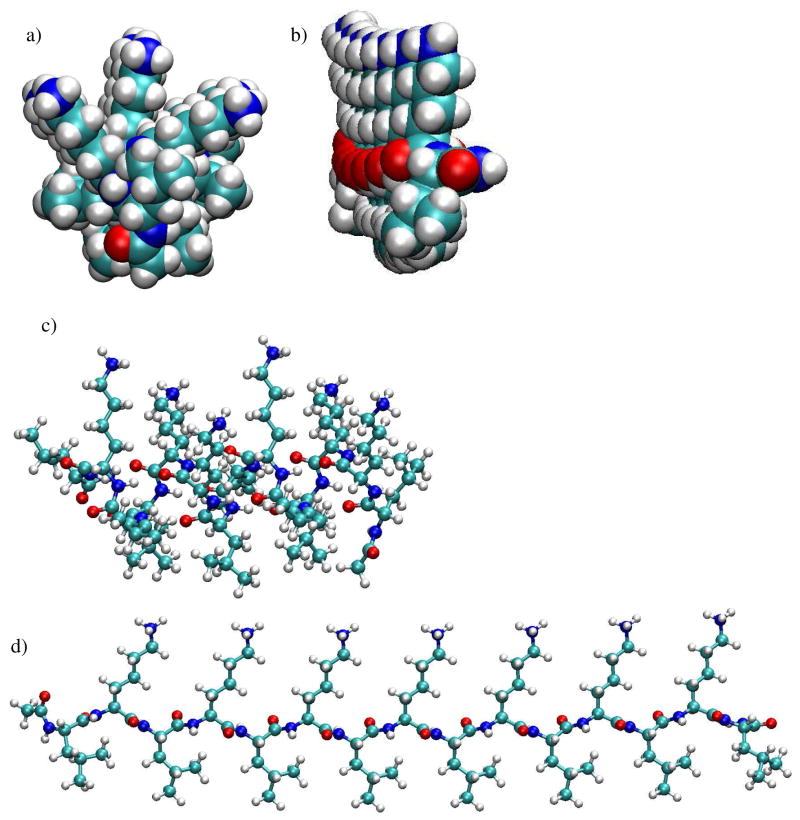
Figure 3.
Minimized starting conformations of LKα14 and LKβ15 models used for molecular dynamic simulations. All figures are shown with the lysine side-chains pointing up and the leucine side-chains pointing down. Nitrogen atoms are dark blue, carbon atoms are light blue, oxygen atoms are red and hydrogen atoms are grey. Figures a and b show space-fill end-on views of a) LK α14, and b) LKβ15. It can be seen that in contrast to well separated lysine and leucine side chains in the LKβ15 structure, the lysine and leucine side chains fan out and reduce the separation between the lysine and leucine side chains in the LKα14 structure. Figures c and d show ball-and-stick side-views of LKα14 and LKβ15, respectively.