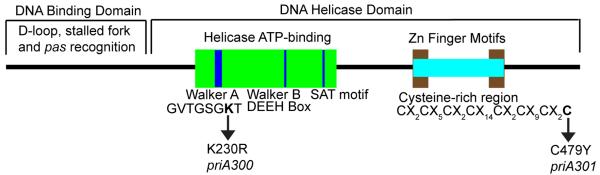
Figure 2.
Amino acid motifs of E. coli PriA. PriA is divided into two major domains, a DNA-binding domain and a DNA helicase domain. The DNA helicase domain consists of an ATP-binding domain composed of a Walker A box, Walker B box, and SAT motif, and a cysteine-rich region that contains two Zn-finger motifs. Amino acid substitutions in the Walker A box, K230R, yielding a helicase-dead mutant protein, and in the metal binding domain, C479Y, representing the priA300 and priA301 mutations, are shown.