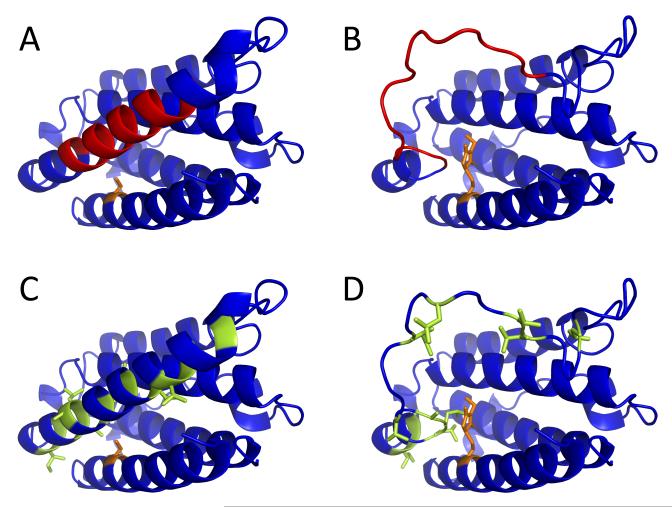
Figure 4.
Location of the [88-102] segment (highlighted in red) within the crystal structure of IFN (1AU1, panel A) with respect to Cys-17 (highlighted in orange). Alkylation of Cys-17 inevitably leads to steric clashes within the native structure, which can be removed by unfolding of the helix D containing the [88-102] segment (panel B). Side chains of hydrophobic residues within helix D are sequestered in the protein interior (highlighted in green, panel C), but become exposed to solvent upon unfolding of this structural element (panel D).