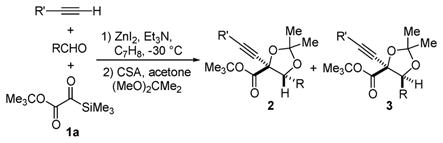
Table 1.
Reaction Scope with 1aa
| ||||||
|---|---|---|---|---|---|---|
| entry | R′ | R | yield (%)b | 2:3c | ||
| 1 | Ph | Ph | 77 | 78:22 | ||
| 2 | n-C6H13 | Ph | 77 | 74:26 | ||
| 3 | BnOCH2 | Ph | 60 | 72:28 | ||
| 4 | PhthNCH2 | Ph | 48 | 71:29 | ||
| 5 | Me3Si | Ph | 81 | 76:24 | ||
| 6 | Me3Si | 2-(Me)C6H4 | 65 | 80:20 | ||
| 7 | Me3Si | 4-(MeO)C6H4 | 73 | 80:20 | ||
| 8 | Me3Si | 4-(Cl)C6H4 | 76 | 78:22 | ||
| 9 | Me3Si | 2-furyl | 66 | 47:53 | ||
| 10 | Me3Si | (E)-PhCHd=CH | 77 | 53:47 | ||
Reaction carried out using 1.0 equiv of 1a, 3.0 equiv of alkyne, 1.5 equiv of PhCHO, 3.0 equiv of ZnI2, and 3.3 equiv of Et3N for 24–48 h.
Average of two isolated yields. All reported yields are for two steps.
Determined by 1H NMR analysis of the crude reaction. See Supporting Information for determination of relative stereochemistry.