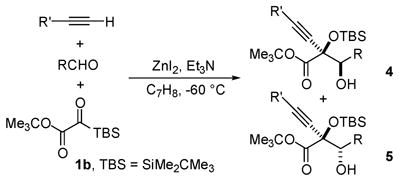
Table 2.
Reaction Scope with 1ba
| ||||||
|---|---|---|---|---|---|---|
| entry | R′ | R | yield (%) | 4:5b | ||
| 1 | Ph | Ph | 73 | 83:17 | ||
| 2 | n-C6H13 | Ph | 73 (53) | 90:10 (>99:1) | ||
| 3 | Me3Si | 2-(Me)C6H4 | 70c | 91:9 | ||
| 4 | n-C6H13 | 4-(MeO)C6H4 | 72c | 90:10 | ||
| 5 | n-C6H13 | 2-(Me)C6H4 | 68c | 92:8 | ||
1.0 equiv of 1b, 4.0 equiv of alkyne, 1.5 equiv of PhCHO, 4.0 equiv of ZnI2, and 4.4 equiv of Et3N for 48 h.
Determined by 1H NMR analysis of the crude reaction.
5.0 equiv of alkyne, 5.0 equiv of ZnI2, and 5.5 equiv of NEt3 used. Parenthetical yield and dr refers to the diol, revealed after TBAF deprotection and recrystallization (two-step yield).