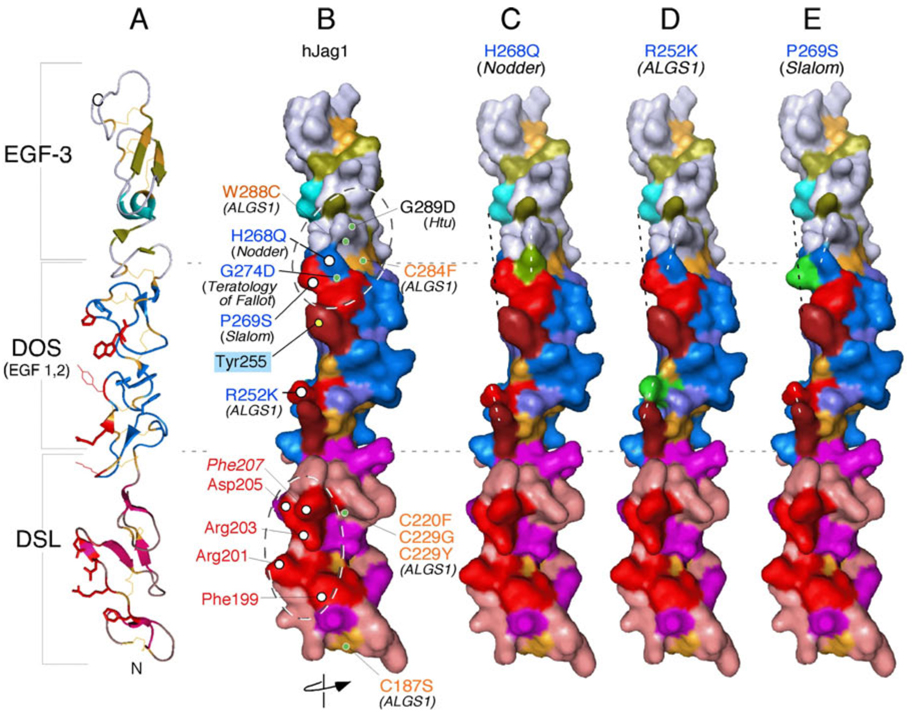
Figure 4.
Mapping known autosomal dominant mutations on the surface of hJagged1 indicates that the DOS domain could form part of the Notch-binding interface. The hJagged1 ribbon structure (A) was surface rendered and rotated such that the Notch-binding interface is facing the reader (B–E). B) Structural model of the wild-type ligand showing biologically relevant residues. Alagille syndrome (ALGS)-associated missense mutations that are likely to affect disulfide bonding (and thus the structural integrity of these domains) are labeled in gold. Additional relevant DSL and DOS domain amino acids are labeled in red and blue, respectively, and further distinguished as being surface-exposed (white circles) or buried within the structure (green circles). A positively charged cluster of highly conserved surface-exposed residues within the DSL domain (labeled in red) identifies a putative Notch-binding surface (see text and Figure 3 legend). Interestingly, missense mutations associated with Teratology of Fallot in humans, and to autosomal dominant inner ear malformations in mice (i.e., headturner (Htu), slalom and Nodder) cluster near a common DOS region. Mutations in headturner and Teratology of Fallot affect amino acids buried under the surface defined by slalom and Nodder mutations and may impact the structure of the potential Notch binding site within DOS. Note that R252 (ALGS1) and Y255 (unique to Jagged; see Figure 3 legend) are also aligned with the putative Notch binding surfaces on the DSL and DOS domains. C–E) Modeling of specific substitutions of surface amino acids (highlighted in green) in the DOS domain results in realignment of the surface (e.g., Nodder and ALGS1; dashed white lines) or perturbations into space (e.g., slalom; dashed black line) that could potentially affect interactions with Notch. The exact topology of Notch/ligand interface remains to be explored by co-crystallization.