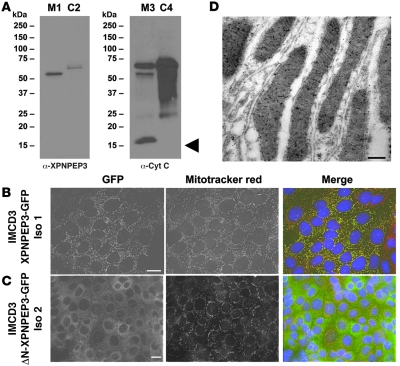
Figure 3. Human XPNPEP3 is targeted to mitochondria by an amino-terminal mitochondrial signal sequence.
(A) Whole kidney homogenates from mice were fractionated into mitochondrial fractions (lanes M1 and M3) and cytosolic fractions (lanes C2 and C4). Immunoblotting with α-XPNPEP3 yielded a single band in the mitochondrial fraction at approximately 51 kDa (lane M1), consistent with the product of cleavage of the mitochondrial signal peptide (after amino acid 53) predicted to occur after mitochondrial import. A doublet was seen in the cytosolic fraction (lane C2) at approximately 57 kDa, which is compatible with the full-length isoform 1 of XPNPEP3. Reblotting with a monoclonal antibody against the mitochondrial protein cytochrome c (Cyt C) demonstrates that cytochrome c is enriched in the mitochondrial fraction (lane M3), yielding a band at approximately 15 kDa (arrowhead), which was absent from the cytosolic fraction (lane C4). (B) Immunofluorescent microscopy was performed on IMCD3 cells that stably express the human full-length XPNPEP3-GFP (in 90% of cells) (left panel), which contains the mitochondrial leader sequence. Upon costaining with the mitochondrial marker, Mitotracker Red, there was colocalization (right panel) in mitochondria. Scale bar: 10 μm. Iso 1, isoform 1. (C) By contrast, in IMCD3 cells that stably express an amino-terminal deletion construct of XPNPEP3-GFP, lacking the 53–amino acid N-terminal mitochondrial signal sequence (ΔN-XPNPEP3-GFP) (left panel), XPNPEP3 expression occurred diffusely in the cytoplasm rather than in mitochondria (right panel). Mitochondria were again labeled with the marker Mitotracker Red. Scale bar: 10 μm. (D) To detect presence of endogenous Xpnpep3 in mitochondria of distal tubular segments in rat kidney, α-XPNPEP3 was labeled with 10-nm gold particles. Endogenous Xpnpep3 labeling was detected in mitochondria of distal tubular segments. Scale bar: 1 μm.