Abstract
The stress response—increases in circulating glucocorticoids following a stressor—is typically considered adaptive, but few studies address the fitness consequences of individual variation in stress response. Generally, due to negative consequences of prolonged elevation of glucocorticoids, animals should have a transient stress response just sufficient to cope with the stressor. In rodents, stress responsiveness is affected by early developmental experience, and hyper-responsiveness to stress is linked to morbidity and mortality. We assessed individual variation in stress responses in free-living song sparrows, Melospiza melodia, in relation to fitness-related measures including song and overwinter survival. Birds with greater increases in corticosterone 30 min following restraint stress were less likely to return to breed the following year. Stress responsiveness was also correlated with song complexity: males with fewer syllables in their song repertoires had greater stress reactivity. Our findings support the hypothesis that developmental stressors both impair song development and affect the adult stress response. Thus, individual variation in the stress response may relate to variation in fitness.
Keywords: birdsong, stress response, survival
1. Introduction
In vertebrates, the endocrine response to stress involves up-regulation of the hypathalamo–pituitary–adrenal (HPA) axis. In response to a perceived stressor, the adrenal cortex increases circulating levels of glucocorticoid hormones (primarily corticosterone in birds) that have numerous effects, including mobilizing energy to allow the organism to cope with a stressor during this emergency life history stage (Wingfield et al. 1998). Because prolonged elevation of glucocorticoids can have detrimental effects (e.g. McEwen 1999), regulation of HPA function is critical. Optimal HPA function should thus involve regulation of both increasing and decreasing levels of glucocorticoids in order to cope with a stressor (Romero 2004).
Despite a general consensus that the stress response is adaptive, few empirical studies have addressed the fitness consequences of individual variation in acute stress response (Breuner et al. 2008). Variation in corticosterone levels has been linked to survival in marine iguanas (Romero & Wikelski 2001) and storks (Blas et al. 2007). However, the relationship between corticosterone levels and fitness may vary with life history stage (Bonier et al. 2009). Further clarifying this relationship requires examining individual variation in the stress response, and in particular the dynamics of HPA function (Romero 2004).
One important factor underlying individual variation in stress reactivity is early developmental exposure to stress. Prolonged perinatal stress can result in increased adult glucocorticoid secretion in mammals (Darnaudéry & Maccari 2008). There is now growing evidence in birds for a similar effect: prolonged stress or corticosterone treatment early in life results in a greater increase in corticosterone during acute stress later in adulthood (Pravosudov & Kitaysky 2006; Spencer et al. 2009). Thus, early developmental experience may have important epigenetic effects through modifying the adult stress response and thereby fitness.
We explored the relationships between the acute stress response, song complexity and overwinter survivorship in song sparrows (Melospiza melodia). In this species, song complexity has been linked to physiological condition (Reid et al. 2005; Pfaff et al. 2007), and reproductive success (Reid et al. 2004). Moreover, nutritional stress during development impairs growth of song-control brain regions (MacDonald et al. 2006). We hypothesized that if male sparrows sing less complex songs due to prolonged exposure to stress during development (Nowicki et al. 1998), then they should also exhibit altered stress responses in adulthood. To address this, we measured baseline and stress-induced levels of corticosterone in free-living sparrows, and related this to their song complexity and overwinter survival.
2. Material and methods
We mist-netted 24 adult male song sparrows breeding near Newboro, Canada, in May and June 2007. We collected a blood sample (baseline) within 3 min of the bird entering the net, then placed the bird in a paper bag. After 30 min we collected a second blood sample (stressed; Clinchy et al. 2004), and released the bird. Blood was kept cool in the field, centrifuged later that day, and supernatant plasma kept frozen until assay. Plasma was assayed for total corticosterone using a specific and sensitive radioimmunoassay kit (ImmuChem 07-120103, MP Biomedicals, Orangeburg, NY, USA; Washburn et al. 2007) previously validated for song sparrows (Newman et al. 2008). All samples were measured in a single assay. Sensitivity of the assay was 12.5 ng ml−1 and within-assay coefficients of variation were 9.6 and 3.9 per cent for low and high controls.
We recorded complete song repertoires of 18 of these males (as validated by Pfaff et al. 2007) using Marantz Professional Solid State PMD 671 recorders and Telinga Twin Science parabolic microphones (Uppsala, Sweden). We generated sound spectrograms in Syrinx 2.6h (J. Burt; www.syrinxpc.com) and counted the total number of song types (song repertoire size) following Pfaff et al. (2007) and total number of syllables (syllable repertoire size) following Stewart & MacDougall-Shackleton (2008). These two measures are highly correlated (r = 0.7), but nonetheless predict different aspects of male phenotype (MacDougall-Shackleton et al. 2009).
We monitored breeding success for the remainder of the 2007 season, and the subsequent year (2008). As an index of territory quality we monitored the number of offspring produced (see electronic supplementary material). Song sparrows are highly philopatric following their first breeding attempt. In our population, males typically nest within 30 m of their nest the previous year. Males who do not return from the wintering grounds are presumed to have died overwinter. We thus assessed whether the birds sampled above did, or did not return to breed the following year.
We used SPSS 16 to run multiple regressions to determine whether song and other variables significantly predicted corticosterone levels. To determine whether stress response predicted overwinter survival, we ran binary logistic regressions to identify variables that were significantly related to returning to breed in 2008.
Glucocorticoids act via two receptor types, with baseline and stress-induced levels acting through different pathways (Romero 2004). However, because we were primarily interested in individual variation in corticosterone regulation (independent of downstream effects), we assessed which variables were significantly related to the change in corticosterone (stress response). We then separately tested what variables were significantly related to absolute levels of baseline and stress-induced corticosterone.
3. Results
The birds exhibited a stress response (increase in corticosterone over 30 min) comparable to that in other studies of breeding song sparrows (see electronic supplementary material, Clinchy et al. 2004; Newman et al. 2008).
(a). Stress response and song
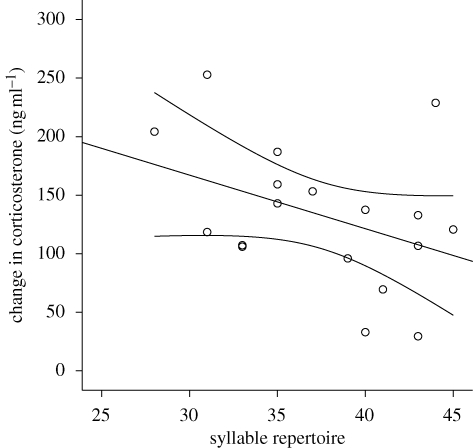
To test whether the stress response was related to song we ran backward and forward multiple regressions to determine which of the following variables significantly predicted the increase in corticosterone (stressed − baseline levels): song and syllable repertoire size, number of offspring in the first nest, body mass and date. Both forward and backward procedures yielded the same model, in which only syllable repertoire size significantly predicted stress response (overall model: r2 = 0.46, p = 0.01). Specifically, birds with smaller syllable repertoires had greater corticosterone increases following restraint than birds with larger syllable repertoires (figure 1; standardized β = −0.68, p = 0.01). Baseline corticosterone was not related to any of these variables; stressed levels of corticosterone were predicted by syllable repertoire size (see electronic supplementary material).
Figure 1.
The relationship between song complexity (syllable repertoire size) and stress response (stress-induced baseline corticosterone levels). Lines indicate least-square linear regression (fitted for purposes of illustration) and 95 per cent confidence intervals.
(b). Stress response and survival
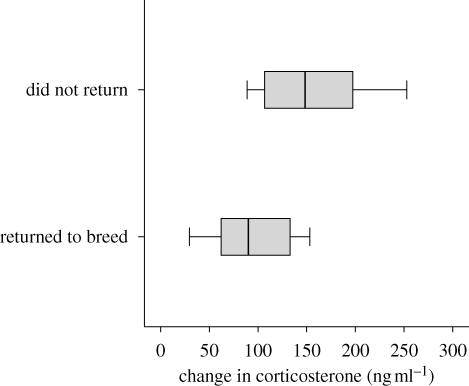
All of the 24 birds for which we measured stress response survived through the 2007 breeding season. Eleven of these males returned to breed in 2008, similar to the typical adult return rate of 45–50% in this population (E. A. MacDougall-Shackleton 2009, unpublished data). Stress responses were highly predictive of which birds returned and which did not: birds that returned to breed in 2008 had significantly lower stress responses in 2007 than those that failed to return (figure 2; Wald = 4.96, n = 24, p = 0.026). Returning to breed in 2008 was also significantly predicted by stress-induced levels of corticosterone (Wald = 4.72, n = 24, p = 0.03). None of the other variables examined predicted survival in this sample (see electronic supplementary material). Thus, a greater stress response to a standardized stressor appears negatively related to survival in this species.
Figure 2.
Probability of returning to breed in 2008, as a function of stress response (stress-induced baseline corticosterone levels), the previous year. Box plots indicate 10th, 25th, 50th, 75th and 90th percentiles.
4. Discussion
We found that (i) birds with less complex songs had greater stress responses, and (ii) birds with greater stress responses were less likely to return to breed the following year. Though observational, these findings suggest important links between courtship signals, the stress response and survival. Variation in corticosterone levels has been linked to survival in other taxa (Romero & Wikelski 2001; Blas et al. 2007). For example, increased juvenile stress responses are predictive of reduced survival to adulthood in storks (Ciconia ciconia; Blas et al. 2007). In humans, hyperreactivity to a cold pressor test predicts hypertension 45–57 years later (Wood et al. 1984). Our findings provide further evidence that stress responses can be predictive of morbidity and mortality.
Although we observed no direct correlation between song complexity and survival, such a relationship has been reported in a western subspecies of song sparrow (Reid et al. 2005). Further analyses are required, but our results suggest that the correlation between song and survivorship may result from both being correlated to a third variable. Perhaps, traits affected by stressors early in life, such as song or the stress response itself become correlated through development. Such developmental correlations may make song a predictor of a wide range of traits associated with direct and indirect benefits. Our study provides evidence of links between a sexually selected ornament, the stress response and survival in a free-living population and suggests that song may act as an indicator of developmental conditions and adult male quality.
Acknowledgements
Elizabeth Hampson, Bavani Rajakumar and the Queen's University Biological Station provided logistical support. Funded by NSERC Canada. All research under permission of Environment Canada and the Animal Use Subcommittee at the University of Western Ontario.
References
- Blas J., Bortolotti G. R., Tella J. L., Baos R., Marchant T. A.2007Stress response during development predicts fitness in a wild, long lived vertebrate. Proc. Natl Acad. Sci. USA 104, 8880–8884 (doi:10.1073/pnas.0700232104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonier F., Moore I. T., Martin P. R., Robertson R. J.2009The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 163, 208–213 (doi:10.1016/j.ygcen.2008.12.013) [DOI] [PubMed] [Google Scholar]
- Breuner C. W., Patterson S. H., Hahn T. P.2008In search of relationships between the acute adrenocortical response and fitness. Gen. Comp. Endocrinol. 157, 288–295 (doi:10.1016/j.ygcen.2008.05.017) [DOI] [PubMed] [Google Scholar]
- Clinchy M., Zanette L., Boonstra R., Wingfield J. C., Smith J. N. M.2004Balancing food and predator pressure induces chronic stress in songbirds. Proc. R. Soc. Lond. B 271, 2473–2479 (doi:10.1098/rspb.2004.2913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnaudéry M., Maccari S.2008Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 57, 571–585 (doi:10.1016/j.brainresrev.2007.11.004) [DOI] [PubMed] [Google Scholar]
- MacDonald I. F., Kempster B., Zanette L., MacDougall-Shackleton S. A.2006Early nutritional stress impairs development of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc. R. Soc. B 273, 2559–2564 (doi:10.1098/rspb.2006.3547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall-Shackleton E. A., Stewart K. A., Potvin D. A., Tennenhouse E.2009The rich get richer: song complexity predicts song element sharing and song output in song sparrows Melospiza melodia. Anim. Behav. 78, 141–146 (doi:10.1016/j.anbehav.2009.04.004) [Google Scholar]
- McEwen B. S.1999Stress and hippocampal plasticity. Ann. Rev. Neurosci. 22, 105–122 (doi:10.1146/annurev.neuro.22.1.105) [DOI] [PubMed] [Google Scholar]
- Newman A. E. M., Pradhan D. S., Soma K. K.2008Dehydroepiandrosterone and corticosterone are regulated by season and acute stress in a wild songbird: jugular versus brachial plasma. Endocrinology 149, 2537–2545 (doi:10.1210/en.2007-1363) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicki S., Peters S., Podos J.1998Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 38, 179–190 [Google Scholar]
- Pfaff J. A., Zanette L., MacDougall-Shackleton S. A., MacDougall-Shackleton E. A.2007Song repertoire size varies with HVC volume and is indicative of male quality in song sparrows (Melospiza melodia). Proc. R. Soc. B 274, 2035–2040 (doi:10.1098/rspb.2007.0170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravosudov V. V., Kitaysky A. S.2006Effects of nutritional restrictions during post-hatching development on adrenocortical function in western scrub-jays (Aphelocoma californica). Gen. Comp. Endocrinol. 145, 25–31 (doi:10.1016/j.ygcen.2005.06.011) [DOI] [PubMed] [Google Scholar]
- Reid J. M., Arcese P., Cassidy A., Hiebert S. M., Smith J. N. M., Stoddard P. K., Marr A. B., Keller L. F.2004Song repertoire size predicts initial mating success in male song sparrows, Melospiza melodia. Anim. Behav. 68, 1055–1063 (doi:10.1016/j.anbehav.2004.07.003) [Google Scholar]
- Reid J. M., Arcese P., Cassidy A., Hiebert S. M., Smith J. N. M., Stoddard P. K., Marr A. B., Keller L. F.2005Fitness correlates of song repertoire size in free-living song sparrows (Melospiza melodia). Am. Nat. 165, 299–310 (doi:10.1086/428299) [DOI] [PubMed] [Google Scholar]
- Romero L. M.2004Physiological stress in ecology: lessons from biomedical research. Trends Ecol. Evol. 19, 249–255 (doi:10.1016/j.tree.2004.03.008) [DOI] [PubMed] [Google Scholar]
- Romero L. M., Wikelski M.2001Corticosterone levels predict survival probabilities of Galapagos marine iguanas during El Niño events. Proc. Natl Acad. Sci. USA 98, 7366–7370 (doi:10.1073/pnas.131091498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K. A., Evans N. P., Monaghan P.2009Postnatal stress in birds: a novel model of glucocorticoid programming of the hypothalamic–pituitary–adrenal axis. Endocrinology 150, 1931–1934 (doi:10.1210/en.2008-1471) [DOI] [PubMed] [Google Scholar]
- Stewart K. A., MacDougall-Shackleton E. A.2008Local song elements indicate local genotypes and predict physiological condition in song sparrows, Melospiza melodia. Biol. Lett. 4, 240–242 (doi:10.1098/rsbl.2008.0010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn B. E., Millspaugh J. J., Morris D. L., Schulz J. H., Faaborg J.2007Using a commercially available enzyme immunoassay to quantify testosterone in avian plasma. Condor 109, 181–186 (doi:10.1650/0010-5422(2007)109[181:UACAEI]2.0.CO;2) [Google Scholar]
- Wingfield J. C., Maney D. L., Breuner C. W., Jacobs J. D., Lynn S., Ramenofsky M., Richardson R. D.1998Ecological bases of hormone–behavior interactions: the ‘emergency life history stage’. Am. Zool. 38, 191–206 [Google Scholar]
- Wood D. L., Sheps S. G., Elveback L. R., Schirger A.1984Cold pressor test as a predictor of hypertension. Hypertension 6, 301–306 [DOI] [PubMed] [Google Scholar]