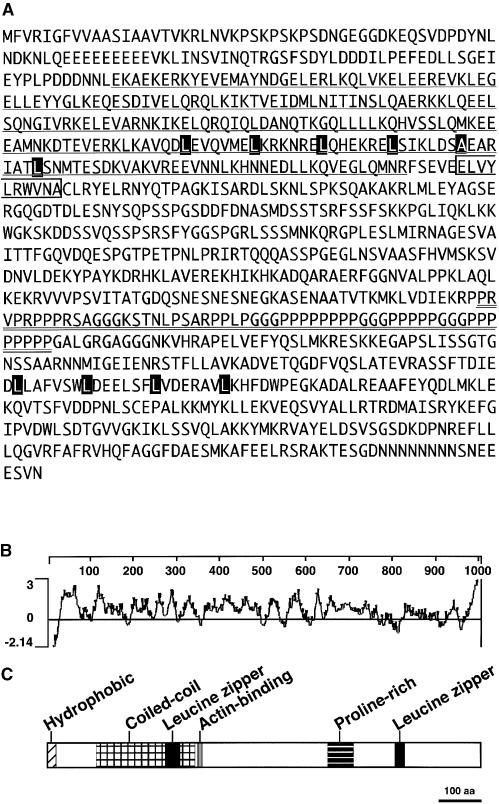
Figure 5.
Structure of CHUP1.
(A) Amino acid sequence of CHUP1. The coiled-coil domain is underlined. The Pro-rich domain is double underlined. The actinin-like actin binding motif is indicated by an open box. Two Leu zipper motifs are indicated by series of black boxes.
(B) Hydrophilicity plot of the amino acid sequence of CHUP1. Hydrophilic indices were calculated using the Kyte-Doolittle scale (Kyte and Doolittle, 1982) and averaging over a window of 17 amino acid residues.
(C) Domain organization of CHUP1. The hydrophobic region, coiled-coil domain, actin binding motif, Pro-rich region, and two Leu zipper domains are indicated. aa, amino acids.