Abstract
The intra-aortic balloon pump has been widely and successfully used as a treatment for cardiac dysfunction, but it only has short-term applications. To overcome this limitation, a superficial counterpulsation device (CPD) is being developed to provide extended counterpulsation support to promote myocardial recovery. The CPD is a valveless, monoport, pneumatically driven, 40-ml sac that is intended to be implanted in a pacemaker-type pocket in the subclavian fossa. The sac is designed to fill in systole and empty during diastole through an outflow graft anastomosed to the subclavian artery. A feasibility study was conducted to investigate acute hemodynamic responses to the CPD in eight calves with diminished cardiac function. The CPD augmented aortic diastolic pressure, reduced left ventricular peak systolic and aortic ejection pressures by up to 18%, and increased diastolic coronary flow by up to 21% and stroke volume by up to 12%. A cadaver fit study demonstrated that the human subclavian artery is a reasonable anastomosis site to consider and that the 40-ml CPD needs to be reduced in size to provide a better anatomical fit. The clinical attractiveness of this approach is that it may provide extended support through a subcutaneous surgical procedure.
The incidence of congestive heart failure (CHF) is increasing worldwide, with more than one million new cases diagnosed annually. Current treatment options for CHF include optimal medical management therapy, mechanical heart assist, and heart transplantation. Optimal medical management therapies treat the symptoms and delay the progression of CHF, and cardiac support devices can restore end-organ perfusion, but these therapies have been largely unsuccessful in curing the disease. Heart transplantation offers the best opportunity for long-term survival; however, the number of available donor organs (~2,500) cannot meet the growing demand (up to 100,000 per year).
Over the past four decades, counterpulsation with an intra-aortic balloon pump (IABP) has been widely used for the short-term treatment of cardiac dysfunction,1,2 with up to 65% successful clinical outcomes.3 Counterpulsation provides important acute hemodynamic benefits for the heart (lower ventricular workload and increased myocardial perfusion) and the peripheral circulation (increased end-organ perfusion), which may make extended application of counterpulsation a very effective treatment for patients with early-stage heart failure.1,2 However, the location of an IABP catheter (in the descending thoracic aorta) and biocompatibility issues limit the application of IABP to short durations. When IABP support was attempted for a prolonged period (>20 days), the frequency of vascular complications, infections, and bleeding were significantly higher.4,5 Furthermore, in its current form, a balloon device mounted on a catheter is typically advanced from a groin artery into the descending aorta, requiring a patient to remain supine for the duration of therapy, which virtually immobilizes the patient. In addition, for extended use, the IABP balloon may not be sufficiently reliable and may wear out as the result of repeated contact with aortic plaque formation. Consequently, the number of patients who could benefit from counterpulsation with IABP as an extended therapy to promote myocardial recovery is limited.
If counterpulsation could be shown to assist in the treatment of early-stage heart failure patients by controlling their symptoms or by slowing the decay in cardiac function, this therapy could potentially be a much simpler and safer treatment than typical mechanical circulatory support devices under development today. A number of devices have been proposed to extend the application of counterpulsation therapy, including the dynamic aortic patch,6,7 valveless counterpulsation device,8 artificial vasculature device,9 and aortic cuff (Sunshine Medical C-Pulse™). However, like ventricular assist devices (VADs), these devices may require major surgery. To overcome these limitations, we propose a counterpulsation device (CPD) for long-term application to treat patients with early-stage heart failure who can be implanted through the use of a superficial surgical approach. The predicted acute hemodynamic benefits of the device were evaluated in previously reported computer and mock circulation models.10 In this article, we report preliminary findings from a feasibility study of a prototype 40-ml CPD tested acutely in calves and a human cadaver anatomical fit study.
Methods
Counterpulsation Device
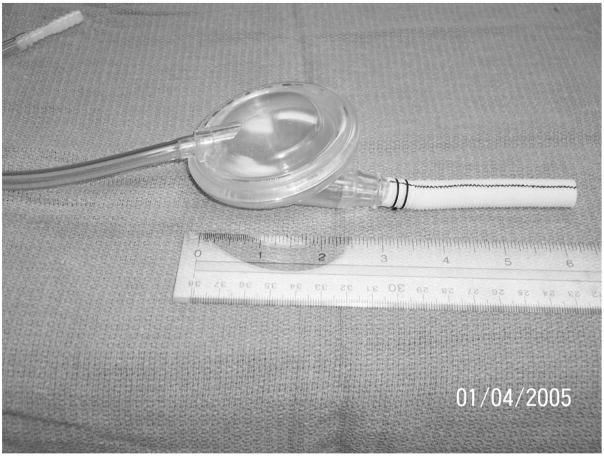
A CPD is being developed for implantation under the pectoral muscle in a pacemaker pocket. The inlet-outlet graft is being designed for anastomosis to the subclavian artery. The subclavian artery is a large vessel that connects with the innominate artery and thus provides a low resistance path to the ascending aorta, eliminating the need for surgical entry into the chest or abdomen. The prototype CPD is a valveless, pneumatically driven, 40-ml sac that fills and empties blood volume through a single cannula (Figure 1). It provides counterpulsation therapy by filling during native heart systole (deflation) and ejecting during native heart diastole (inflation). Volume displacement in the sac is controlled by a pneumatic driver delivering metered pulses of compressed air to the pressurization chamber during ventricular diastole. Currently, inflation and deflation timing and period are manually adjusted to augment aortic diastolic pressure and reduce after-load.
Figure 1.
Photograph of a 40-ml counterpulsation device (CPD). The CPD sac (1) fills with 40-ml blood volume during ventricular systole and empties during ventricular diastole through a single, valveless cannula (2). The diaphragm is pneumatically driven by controller through an air line (3).
Animal Experiments
A prototype 40-ml CPD was tested acutely in calves with diminished cardiac function (DCF). To create the DCF model, calves were given a single dose of Monensin to achieve an appropriate degree of moderate heart failure defined by 20% reduction in cardiac output and an elevated central venous pressure of 10 to 12 mm Hg.11 In initial experiments, the CPD was cannulated to the brachiocephalic artery (n = 4) to mimic cannulation to the human subclavian artery (8 to 10 mm diameter). In subsequent experiments, the CPD device was cannulated to the carotid artery (5 to 7 mm diameter, n = 4) to mimic a more peripheral circulation. Hemodynamic waveforms were recorded during (1) baseline, (2) CPD in 1:1 mode, and (3) CPD in 1:2 mode. Baseline measurements were repeated between test conditions to ensure that baseline values were maintained.
Surgical Procedures
Eight healthy, male, Jersey breed calves (55 to 90 kg body weight) were used in this study. After initial physical examination and a 5- to 7-day quarantine period, arterial and venous pressure monitoring lines were implanted. The calves were given Atropine (30 mg subcutaneously) and then anesthetized with isoflurane (5%) through a nose cone. Calves were then intubated and anesthesia was maintained with isoflurane (1% to 3%) mixed in oxygen and room air. The left lateral neck of the animal was prepared for sterile surgery. A 5-cm incision was made dorsal to the jugular vein.
Flocked polypropylene catheters were inserted into the right carotid and right external jugular vein and then tunneled under the skin to exit near the dorsum of the neck. The skin incision was then closed in two layers and the calves were allowed to recover. Banamine (100 mg subcutaneously every 6 hours) was given for analgesia for the first 48 hours. Cefazolin (1.0 g intravenously every 8 hours) was given for prophylaxis for the first 3 days after surgery.
Baseline arterial and venous pressure measurements were made over a 5- to 7-day period, before Monensin was given. After a 5- to 7-day recovery period, the calves were given a single oral dose of Rumensin 80 equivalent to 20 mg/kg (Elanco, Indianapolis, IN) through an orogastric tube. After administration, calves were given Cefazolin (1.0 g intravenously every 8 hours) for prophylaxis and Banamine (100 mg subcutaneously as needed) for analgesia.
Approximately 1 week after the administration of Monensin, a nonsurvival acute hemodynamic study with the CPD was done. Animals were preanesthetized with Atropine (30 mg) and then anesthetized with isoflurane (3% to 5%) through a nose cone. After intubation, anesthesia was maintained with isoflurane (1% to 3%) mixed with oxygen and room air. The left lateral chest was prepared for aseptic surgery. A thoracotomy was done over the third and fourth ribs, which were resected. Animals were anticoagulated with heparin (3.0 mg/kg), and activated clotting times were monitored and maintained for >300 seconds. Ultrasonic flow probes were set around the pulmonary artery, descending aorta, brachiocephalic trunk, left main coronary artery, and CPD inflow/out-flow graft. Pressure monitors (Millar Instruments, Houston, TX) were placed in the left atrium and brachiocephalic trunk. A dual-pressure volume conductance catheter was advanced from the aorta into the left ventricle. The CPD was either attached onto the brachiocephalic artery with a 24F aortic cannula or anastomosed to the carotid artery by means of a 10-mm vascular graft. A photograph of the complete surgical model, including instrumentation and CPD placement, is shown in Figure 2. After completion of experimental protocol, animals were euthanized by increasing the anesthetic depth (isoflurane 5%) and given a bolus injection of supersaturated KCl (20 ml intravenously).
Figure 2.
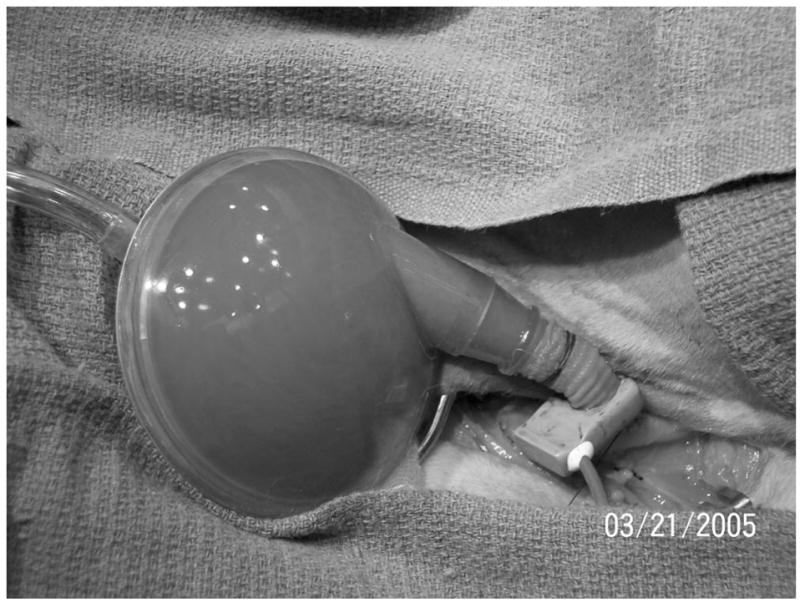
Photograph of experimental setup for acute testing of the 40-ml counterpulsation device (CPD) in the diminished cardiac function (DCF) calf model. A pressure-volume conductance catheter (Millar Instruments, Houston, TX) measured simultaneous left ventricular pressure, aortic pressure, and left ventricular volume. Single-tip, high-fidelity catheters (Millar Instruments, Houston, TX) measured left atrial and pulmonary artery pressures. Transit-time flow probes (Transonics, Ithaca, NY) measured aortic, pulmonary artery, brachiocephalic, left main coronary artery, and CPD flows. The CPD is a 40-ml sac with a single, valveless cannula anastomosed to the carotid artery to mimic subclavian cannulation in humans. The CPD was timed to fill with during native heart systole and eject during native heart diastole.
All animals received humane care in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86–23, revised 1996) and the guidelines determined by the Institutional Animal Care and Use Committee of the University of Louisville (protocol No. 04114, approved November 18, 2004).
Hemodynamic Analysis
All transducers were precalibrated and postcalibrated against known standards to ensure measurement accuracy. Hemodynamic data were signal-conditioned and analog-to-digitally converted at a sampling rate of 400 Hz and stored for digital analysis by using a Good Laboratory Practices–compliant data acquisition system.12,13 Pressure, flow, and volume waveforms were used to calculate landmark cardiovascular function parameters (i.e., heart rate, stroke volume, cardiac output, and mean, systolic, diastolic, peak positive and peak-negative pressures and flows), using a Hemodynamic Evaluation and Assessment Research Tool (HEART) program14 developed in Matlab (MathWorks, Natick, MA). Hemodynamic parameters were calculated on a beat-to-beat basis, with all beats in each data set averaged to obtain a single representative mean value. Left ventricular pressure-volume (PV) loops were constructed by plotting ventricular pressure against ventricular volume, with each loop representing one complete cardiac cycle (one beat). These PV loops were used to calculate external work and to determine reduction in ventricular filling and/or ejection pressures and changes in ventricular end-diastolic, end-systolic, and stroke volumes.
Cadaver Fit Study
An approved anatomical fit study was performed with a 70-kg human cadaver from the Fresh Tissue Laboratory at the University of Louisville to assess the surgical approach and positioning of the CPD. A pacemaker-type pocket was created above the right pectoral muscle. The subclavian artery was dissected and exposed. The outflow graft was attached with two simple sutures to the outside of the subclavian artery to simulate an anastomosis. The graft was cut to length and then attached to the CPD sac, which was then placed in the prepectoral pocket. The driveline was tunneled subcutaneously, then exteriorized approximately 15 cm caudal to the pocket, at the lower end of the thoracic cage. The skin was closed over the sac.
Results
Cardiovascular Hemodynamics
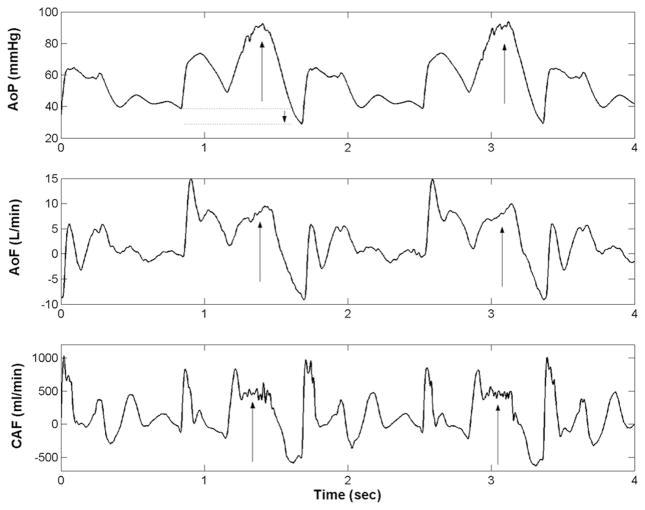
The hemodynamic effect of counterpulsation on waveform morphology (augmented aortic diastolic pressure and reduced ventricular ejection pressures) was observed in all test subjects (Figure 3). Means and standard errors of landmark hemodynamic parameters are presented in Table 1. Compared with baseline, the CPD reduced ventricular workload, as evidenced by reductions up to 18% in left ventricular and aortic ejection pressures. The CPD also increased mean diastolic coronary artery flow by up to 21% and LV stroke volume by up to 12% compared with baseline.
Figure 3.
Illustration of hemodynamic benefits of 40-ml counterpulsation device (CPD) device in calf with diminished cardiac function (DCF). The CPD is operating in 1:2 mode, where every other beat the CPD provides counterpulsation. As indicated by arrows, the CPD increases aortic diastolic pressure (AoP), descending aortic flow (AoF), and diastolic coronary artery flow (CAF). The CPD also decreases afterload, as seen by dotted lines and downward arrow in the AoP waveform.
Table 1.
Summary of Key Acute Hemodynamic Benefits of 40-ml Counterpulsation Device Therapy in the Diminished Cardiac Function Calf Model
| Test Condition | LVEW (mm Hg/ml) | LVPpk-sys (mm Hg) | AoP Eject (mm Hg) | CAF (ml/min) | SV (ml) |
|---|---|---|---|---|---|
| Baseline | 7806 ± 1098 | 110 ± 4 | 101 ± 4 | 158 ± 11 | 89 ± 11 |
| CPD 1:1 | 8104 ± 1032 | 102 ± 6 | 94 ± 6 | 165 ± 23 | 96 ± 11 |
| CPD 1:2 | 7770 ± 1125 | 103 ± 6 | 95 ± 5 | 166 ± 25 | 91 ± 12 |
Values are mean ± standard error.
LVEW, Left ventricular external work; LVPpk-sys, left ventricular peak systolic pressure; AoP Eject, mean aortic pressure during ejection; CAF, mean diastolic coronary artery flow; and SV, stroke volume.
These data suggest that the CPD has the physiological benefits of reducing ventricular workload and augmenting myocardial perfusion.
Left Ventricular Pressure-Volume Loops
The CPD lowered ventricular end-systolic and end-diastolic volumes and lowered ventricular ejection pressure as seen in the left ventricular pressure-volume loops (Figure 4).
Figure 4.
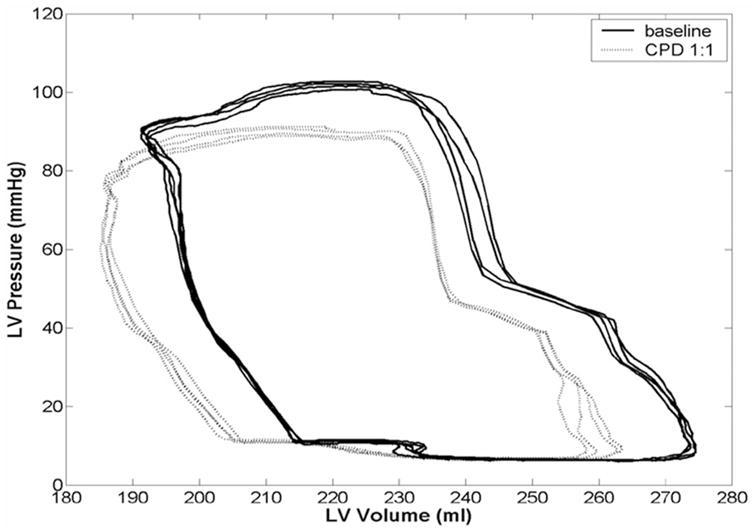
Left ventricular pressure-volume (PV) relation in diminished cardiac function (DCF) calf model at baseline (solid line) and with 40-ml counterpulsation device (CPD) device (dotted line). The CPD reduces end-systolic and end-diastolic volumes compared with failure baseline, resulting in a leftward shift of the PV loops. The CPD also reduces left ventricular ejection pressure, demonstrating another important feature of counterpulsation therapy, which provides pressure unloading while enabling the heart to fill and eject at normal volumes.
Cadaver Fit Study
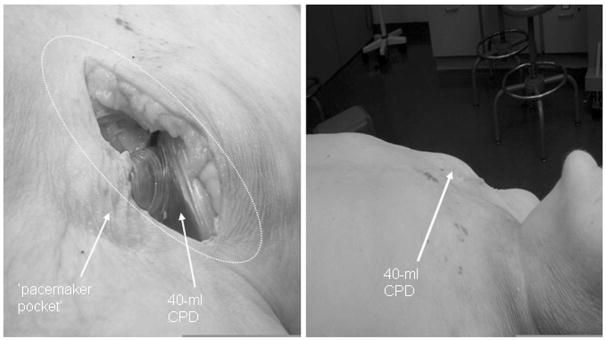
The anatomical fit study suggested that the 40-ml CPD may be successfully positioned in large adults (Figure 5, left), but a smaller-sized device would be more advantageous, particularly in small men and women. When the skin was closed, there was a significant elevation of the right shoulder region and some tension along the line of skin closure (Figure 5, right).
Figure 5.
Photographs from cadaver fit study to demonstrate feasibility of concept of minimally invasive “pacemaker pocket” surgical procedure (left) to implant 40-ml CPD. There was bulging of the pocket in this large male (right), which may suggest that a smaller device may be more desirable, especially in smaller men and women.
Discussion
There is considerable clinical evidence to suggest that counterpulsation may be effective in supporting patients in CHF. Cochran et al.15 reported the use of IABP in patients awaiting transplantation, in which a standard balloon pump was inserted into the aorta through a graft sewn onto the subclavian artery. The majority of these patients demonstrated a decrease in pulmonary capillary wedge pressure and an increase in cardiac output. Furthermore, the ventricular benefit of counterpulsation was studied in patients with poor cardiac function undergoing coronary artery bypass surgery. Using an intraventricular conductance catheter, a clear, instantaneous shift in the PV loop of patients who received intra-aortic balloon counterpulsation was demonstrated.16 It is important to note that the improvement in cardiac function occurred immediately. It is not unreasonable to suggest that additional benefit may occur over time in these patients. Since many patients with CHF have low cardiac output, coronary perfusion may be impaired, which may result in further deterioration in cardiac function. Prolonged (days to weeks) improvement in coronary flow may lead to additional secondary improvement in cardiac function.
The findings of this feasibility study support the concept of counterpulsation therapy by volume displacement to provide acute hemodynamic benefits of lower ventricular workload and vascular afterload and augmented myocardial perfusion. In computer simulation and mock circulation models, our group demonstrated that hemodynamic changes varied with inflation and deflation timing, duration, and profile.10 In developing a myocardial rehabilitation program, control of CPD timing and volume will be critical.
The primary hemodynamic advantages of “pressure unloading” using counterpulsation therapy is that it reduces ventricular workload and augments myocardial perfusion while enabling the heart to fill and empty over a normal range of end-systolic and end-diastolic ventricular volumes. As shown in the acute animal experiments, counterpulsation therapy may provide up to 10% to 15% improvement in hemodynamic function. Clearly, this level of support is inadequate for end-stage heart disease but probably is sufficient to successfully treat patients with earlier-stage heart failure. The acute and long-term physiologic benefits associated with these hemodynamic improvements warrant investigation.
Subsequently, it is our hypothesis that application of extended counterpulsation therapy in early-stage heart failure will provide patients sufficient hemodynamic support to meet metabolic needs while enabling the heart to function over a normal range of ventricular volumes (ΔV). By contrast, VADs “volume unload” the heart, restoring end-organ perfusion to normal ranges to meet the metabolic demands of the body. These devices provide substantial hemodynamic improvement by restoring cardiac output to normal ranges. However, as the level of VAD support is increased, variation in ventricular end-systolic and end-diastolic volume (ΔV) may drastically diminish, especially with continuous volume unloading VADs. The physiologic responses to long-term continuous volume unloading have not been completely characterized.
The concept of subcutaneous counterpulsation device placement is quite simple: Instead of opening the chest to cannulate the aorta, the subclavian artery to innominate artery pathway is used as a low-resistance path back to the aorta. The proposed surgical technique is minimally invasive. In humans, the subclavian artery is approximately 8 to 10 mm in diameter, where it will be superficially exposed. It is expected that this vessel size will be adequate to perform counterpulsation from that location. DeBakey17 reported on two patients supported with a pulsatile left ventricular assist device, where the outflow graft was end-to-side anastomosed to the right axillary artery, which is even more peripheral than the right subclavian artery anastomosis site that we are proposing. Both patients were effectively supported for several days, until they could be weaned from the device and were ultimately discharged and became long-term survivors. There were no adverse effects reported with respect to the peripheral cannulation of the flow returning to the right axillary artery.
The cadaver fit study demonstrated that the 40-ml sac produced a visible distortion of the skin and also produced some tension on the line of closure for the incision. In large-sized patients, this may not be a problem. However, for smaller patients, this may be uncomfortable and may also result in a risk of dehiscence of the closure. As a result of these studies, a smaller, 32-ml sac has been produced and is undergoing reliability and performance testing. The graft angle and the gas line exit were also adjusted in this device to improve the fit of this device. This device will provide a much better fit in adult patients.
Counterpulsation has been used extensively to treat short-term episodes of CHF, but little progress has been made toward the long-term therapeutic potential of this therapy. If counterpulsation could be shown to assist in the treatment of patients with CHF by controlling their symptoms or by slowing the decay in cardiac function, this therapy could potentially be a much simpler and safer treatment than typical circulatory assist devices under development today. In recent years, single-outlet, valveless auxiliary ventricles adapted from artificial heart technology have been used to further explore the effectiveness of an implantable blood pump to provide diastolic counterpulsation for left heart failure. In 1986, initial efforts by Nanas and colleagues18 demonstrated that a 100-ml stroke volume device attached to the abdominal aorta could provide effective support compared to the IABP. They later demonstrated improved support when their counterpulsation device was attached by a short vascular graft to the ascending aorta.19 Continuing with this approach, they recently reported that a 30-ml counterpulsation device attached by a short vascular graft to the ascending aorta and controlled by an IABP console was more effective than a 40-ml IABP in a porcine model of experimental heart failure.20 Most recently, Riebman and colleagues21 have demonstrated that a 10-ml stroke volume counterpulsation device was able to rectify hemodynamics better than a 7-ml pediatric IABP in a piglet model of infant left ventricular failure. In short, there is evidence that counterpulsation is more effective when it is performed with a counterpulsation sac than with an intra-aortic balloon of the same or larger size.
Although counterpulsation is less effective at restoring hemodynamics than other mechanical circulatory support devices, the superficial application of this therapy may allow for much earlier treatment. It is likely that a short general anesthetic will be necessary to carry out this procedure (because of subclavian anastomosis), but the level of invasiveness should be quite similar to a standard pacemaker procedure in terms of patient healing and recovery. It is possible that this simple treatment may be innocuous enough that it can be widely applied to patients at a much earlier stage of heart failure, especially when powered by a small portable driver. A less powerful therapy that is applied at an earlier stage of heart failure may be sufficient to improve cardiac function or at least attenuate the decay that so commonly occurs.
Limitations
The goal of this feasibility study was to investigate the potential acute hemodynamic benefits and present potential clinical advantages of a counterpulsation device and subcutaneous surgical implantation procedure. The experimental data presented in the manuscript were intended to provide preliminary insights into the feasibility of the CPD concept as a potential therapy for early-stage heart failure. The animal experiments were not part of a rigorous experimental design. For example, the CPD cannula was anastomosed to the brachiocephalic (n = 4) and carotid arteries (n = 4), and the cannula material and length were varied. In the last set of four experiments, the cannula was shortened to minimal length (<2 cm) to reduce inertial and time delay effects and specific cannula selected to prevent cannula collapse and thromboembolic events. Subsequently, the hemodynamic benefits were more pronounced in the latter experiments. The investigators did not measure myocardial oxygen consumption in any of the experiments limiting the interpretation of ventricular workload. Despite these limitations, the data demonstrate sufficient feasibility of this CPD approach to warrant more rigorous scientific evaluation.
Conclusion
In summary, the feasibility study of a novel 40-ml counter-pulsation device demonstrated hemodynamic improvements, as evidenced by augmented aortic diastolic pressure, reduced ventricular and aortic ejection pressures, and augmented diastolic coronary flow and stroke volume. The next series of acute experiments are ongoing and include comparison of a 32-ml CPD to a 40-ml IABP clinical system, additional hemodynamic measurements (i.e., myocardial oxygen consumption), and larger sample size to achieve statistically meaningful results in a well-defined and controlled experimental design. Initial chronic evaluation of this CPD approach has also been initiated.
Acknowledgments
This project was funded by a grant from the Whitaker Foundation (RG-01-0310) and the Rudd Research Foundation (Jewish Hospital, Louisville, KY).
References
- 1.Nanas JN, Moulopoulos SD. Counterpulsation: historical background, technical improvements, hemodynamic and metabolic effects. Cardiology. 1994;84:156–167. doi: 10.1159/000176394. [DOI] [PubMed] [Google Scholar]
- 2.Papaioannou GT, Stefanadis C. Basic principles of the intraaortic balloon pump and mechanisms affecting its performance. ASAIO J. 2005;51:296–300. doi: 10.1097/01.mat.0000159381.97773.9b. [DOI] [PubMed] [Google Scholar]
- 3.Torchiana DF, Hirsch G, Buckley MJ, et al. Intraaortic balloon pumping for cardiac support: trends in practice and outcome, 1968 to 1995. J Thorac Cardiovasc Surg. 1997;13:758–764. doi: 10.1016/S0022-5223(97)70235-6. [DOI] [PubMed] [Google Scholar]
- 4.Freed PS, Wasfie T, Zado B, et al. Intraaortic balloon pumping for prolonged circulatory support. Am J Cardiol. 1988;61:554–557. doi: 10.1016/0002-9149(88)90763-1. [DOI] [PubMed] [Google Scholar]
- 5.Manord JD, Garrard CL, Mehra MR, et al. Implications for the vascular surgeon with prolonged (3 to 89 days) intraaortic balloon pump counterpulsation. J Vasc Surg. 1997;26:511–516. doi: 10.1016/s0741-5214(97)70044-2. [DOI] [PubMed] [Google Scholar]
- 6.Schraut W, Kiso I, Freed P, et al. Permanent in-series cardiac assistance with the dynamic aortic patch: blood-prosthesis interaction in long-term canine experiments. Surgery. 1976;79:193–201. [PubMed] [Google Scholar]
- 7.Jeevanandam V, Jayakar D, Anderson AS, et al. Circulatory assistance with a permanent implantable IABP: initial human experience. Circulation. 2002;24:I-183–I-188. [PubMed] [Google Scholar]
- 8.Nanas JN, Lolas CT, Charitos CE, et al. A valveless high stroke volume counterpulsation device restores hemodynamics in patients with congestive heart failure and intractable cardiogenic shock awaiting heart transplantation. J Thorac Cardiovasc Surg. 1996;111:55–61. doi: 10.1016/s0022-5223(96)70401-4. [DOI] [PubMed] [Google Scholar]
- 9.Giridharan GA, Ewert DL, Pantalos GM, et al. Left ventricular and myocardial perfusion responses to volume unloading and afterload reduction in a computer simulation. ASAIO J. 2004;50:512–518. doi: 10.1097/01.mat.0000136513.21369.75. [DOI] [PubMed] [Google Scholar]
- 10.Giridharan GA, Koenig SC, Pantalos GM, et al. Predicted hemodynamic benefits of counterpulsation therapy using a superficial surgical approach. ASAIO J. 2006;52:39–46. doi: 10.1097/01.mat.0000196522.29376.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Litwak KN, McMahan A, Lott KA, et al. Monensin toxicosis in the bovine calf: a large animal model of cardiac dysfunction. Comp Med. 2005;44:45–49. [PubMed] [Google Scholar]
- 12.Drew GA, Koenig SC. Biomedical Patient Monitoring, Data Acquisition, and Playback with LabVIEW®. In: Olansen JB, Rosow E, editors. Virtual Bio-Instrumentation: Biomedical, Clinical, and Healthcare Applications in LabVIEW®. Prentice Hall; 2002. pp. 180–186. [Google Scholar]
- 13.Koenig SC, Woolard C, Drew GD, et al. Integrated data acquisition system for medical device testing and physiology research in compliance with Good Laboratory Practices. Biomed Instr Tech. 2004;38:229–240. doi: 10.2345/0899-8205(2004)38[229:IDASFM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder MJ, Perrrault B, Ewert DL, et al. HEART: an automated beat-to-beat cardiovascular analysis package using Matlab™. Comp Biol Med. 2004;34:371–388. doi: 10.1016/S0010-4825(03)00087-8. [DOI] [PubMed] [Google Scholar]
- 15.Cochran RP, Starkey TD, Panos AL, et al. Ambulatory intraaortic balloon pump use as bridge to heart transplant. Ann Thorac Surg. 2002;74:746–751. doi: 10.1016/s0003-4975(02)03808-0. [DOI] [PubMed] [Google Scholar]
- 16.Schreuder J, Maisano F, Castiglioni A, et al. Closed-Loop intra-aortic counterpulsation in patients with marked arrhythmia using a real-time dicrotic notch prediction algorithm. Am J Cardiol. 2002;90(suppl 6A):44H. [Google Scholar]
- 17.DeBakey ME. Left ventricular bypass pump for cardiac assistance. Am J Cardiol. 1971;27:3–11. doi: 10.1016/0002-9149(71)90076-2. [DOI] [PubMed] [Google Scholar]
- 18.Nanas JN, Mason JW, Taenaka Y, et al. Comparison of an implantable abdominal aortic counterpulsation device with intraaortic balloon pump in a heart failure model. J Am Coll Cardiol. 1986;7:1028–1035. doi: 10.1016/s0735-1097(86)80220-0. [DOI] [PubMed] [Google Scholar]
- 19.Nanas JN, Nanas SN, Charitos CE, et al. Hemodynamic effects of a counterpulsation device implanted on the ascending aorta in severe cardiogenic shock. Trans Am Soc Artif Organs. 1988;34:229–234. [PubMed] [Google Scholar]
- 20.Terrovitis JV, Charitos CE, Tsolakis EJ, et al. Superior performance of a paraaortic counterpulsation device compared to the intraaortic balloon pump. World J Surg. 2003;27:1311–1316. doi: 10.1007/s00268-003-6928-5. [DOI] [PubMed] [Google Scholar]
- 21.Riebman JB. Mechanical assistance for the failing pediatric heart: a new miniaturized counterpulsation device. ASAIO J. 2004;50:128. Abstract. [Google Scholar]