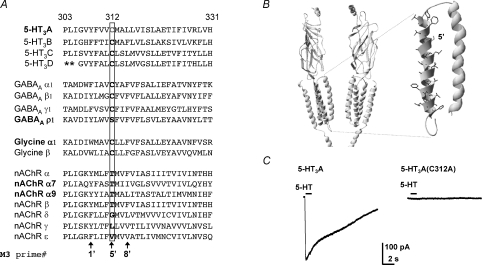
Figure 1. The mutant 5-HT3A(C312A) subunit does not form functional receptors.
A, amino acid sequence alignment of the M3 of human Cys-loop receptor subunits. Note that the human 5-HT3A Cys (C) 312 residue, labelled by shading (grey in print, red online) and boxed, is highly conserved in 5-HT3 subunits, GABAAα, β and γ subunits, and glycine α and β subunits. A prime numbering system is used (De Rosa et al. 2002) to identify homologous M3 residues across all Cys-loop receptor subunits. Asterisks indicate a poorly aligned region of the 5-HT3B subunit sequence, which is omitted for clarity. B, a ribbon rendering of a homology model of the 5-HT3D receptor based on the T. marmorata nACh receptor cryo-EM structure (Unwin, 2005). Two subunits are shown each with four transmembrane domains, a large extracellular N-terminus, the top portion of the cytoplasmic membrane associated helix, and a short C terminal tail. Inset, the M2 and M3 helices expanded with M3 residue side chains rendered and Cys-312 shaded (grey in print, red online). C, representative currents recorded from HEK293 cells transiently expressing WT 5-HT3A or mutant 5-HT3A(C312A) receptors before, during and after 5-HT (100 μm) application for 1 s (where indicated by the bar) at a holding potential of −60 mV. Each trace is a representative of at least eight recordings.