Abstract
The use of hyperthermia as an adjunct to cancer immunotherapy is supported by an increasing number of research data. Both preclinical and clinical data results have demonstrated improved antitumor immune responses with the addition of mild hyperthermia. The molecular mechanisms responsible for the improved immune reactivity observed in the presence of hyperthermia include the generation of Hsps, the activation of antigen presenting cells and changes in lymphocyte trafficking. Understanding these hyperthermia-induced processes can serve as the foundation for analyzing current clinical trials, as well as designing future trials in cancer immunotherapy.
Keywords: hyperthermia, Hsp, immunotherapy, cancer, dendritic cells, immunotherapy
Introduction
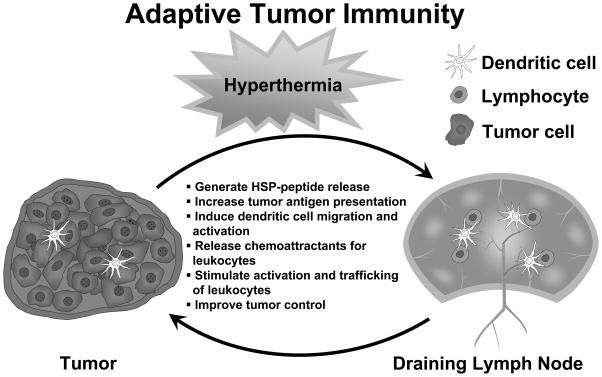
Elevated body temperatures have been recognized as beneficial components of the defense immune response against pathogenic stimuli since ancient times [1], with the notion of treating human cancers with heat dates back to the writings of Hippocrates [2]. However, heat as a treatment modality for cancer has only begun to be evaluated rigorously over the past few decades [2–4]. There is a renewed interest in the application of heat to enhance the efficiency of standard cancer therapies, such as chemotherapy and radiation treatment [5–7]. The combination of immunotherapy with hyperthermia for treating cancer, however, is a particularly intriguing notion, as significant clinical effects of hyperthermia have been attributed to the immune system [8]. The accepted view of the cancer-host immune interface is that tumors possess unique antigens that can be recognized by the immune system. After antigen uptake at tumor sites, APCs have the ability to create a robust response by entering lymphoid compartments and programming lymphocytes. Following generation and expansion to large numbers, cytotoxic lymphocytes may then traffic to tumor sites for targeted cell killing [9], as depicted in Figure 1.
Figure 1. Improvement of antitumor adaptive immunity with hyperthermia.
Hyperthermia impacts the ability of several factors to improve adaptive immunity to tumor antigens culminating in antigen-targeted antitumor responses. DC dendritic cell.
To understand how temperature may influence the immune system, it is necessary to define the concept of hyperthermia. As the father of clinical thermometry, Wunderlich is credited with defining normal body temperatures at 37°C and describing a dynamic range of normal body temperatures with diurnal variations [10]. Fever induces the elevation of the physiological set point of body temperature, increasing core body temperatures via specific thermoeffectors. Hyperthermia differs fundamentally from fever in that it elevates the core body temperature without changing the physiological set point. Typically, hyperthermia is induced by increasing the heat load and/or inactivating heat dissipation [11].
Early studies in hyperthermia focused upon the cytotoxic effects of high temperatures and the direct killing of tumor cells [2]. Although significant cell killing could be achieved by heating cells or tissues to temperatures > 42°C for 1 or more hours, the application, measurement and consistency of this temperature range within the setting of cancer clinical trials proved problematic. Unless thermal ablation of tumor tissue is applied within a localized area, hyperthermia in the cytotoxic range could not be reliably achieved in tumors of heterogeneous size and tissue type [2]. Accordingly, mild temperature hyperthermia (ie, within the fever-range, 39–41°C) and moderate hyperthermia (41°C) have emerged as focal points for ongoing clinical investigations, as they are readily achievable and tolerated [2, 12]. This review delineates aspects of fever-range to moderate-level hyperthermia, and discusses the application of hyperthermia as an adjunct to immunotherapy, focusing on Hsps, and APCs and the enhancement of immunotherapy strategies.
Hyperthermia-induced Hsps as modulators of the immune system
Cellular functions of Hsps
Hsps are a family of stress-induced proteins with several critical cellular functions, and are typically designated by their molecular weight. Hsps were discovered in 1962 as a result of the accidental application of thermal stress to Drosophila preparations [13]. Over the last 30 years, Hsps have been characterized in a variety of cells, in species ranging form prokaryotes to humans, and are highly conserved. Hsps attenuate the effects of cell stressors and were originally assigned a chaperone function, as they prevented intracellular protein misfolding and aggregation during stress responses [14]. Hsps are now recognized as central mediators of a variety of cellular functions under physiological conditions, as they are key regulators of cellular protein activity, turnover and trafficking [15]. During homeostasis, Hsps ensure appropriate post-translational protein folding, and are able to refold denatured proteins, or mark irreversibly damaged proteins for destruction [16]. Unsurprisingly, given their crucial cellular activities, Hsps represent one of the more abundant protein types in unstressed cells, accounting for 1–2% of all cytosolic proteins [17]. Defining the function of Hsps in cancer cells has become a burgeoning area of research, as described below.
Hsps in cancer
The activities of Hsps in transformed tumor cells are complicated and diverse. Hsps are present in an abundance of tumor types and may function to confer several survival benefits to cancer cells [15]. There is evidence that a specific Hsp, Hsp70, directly inhibits apoptosis pathways in cancer cells, as demonstrated in human pancreatic, prostate and gastric cancer cells [18–20]. Hsps have also been implicated in mediating resistance to potentially cytotoxic hyperthermia in a process termed thermotolerance [21–23]. More specifically, the synthesis and accumulation of Hsps in tumor cells exposed to hyperthermia may afford protection from further heat-associated cytotoxic events, as the Hsps may rescue or restore vital cellular proteins. Thermotolerance has the ability to generate a population of tumor cells that are refractory to subsequent hyperthermic changes. Moreover, there is evidence that Hsps support the malignant phenotype of cancer cells by not only affecting the cells’ survival, but also participating in angiogenesis, invasion, metastasis and immortalization mechanisms [24]. Contrary to the many benefits conferred upon tumor cells expressing high levels of Hsps, tumor cell dependence upon Hsps for several critical functions represents an attractive and potential therapeutic target [25]; a virtual Achilles’ heel.
Hsps and the immune system
The immune system has evolved to take advantage of the ability of Hsps to act as ‘danger signals’, thus allowing the generation of an amplified immune response [26]. Hsps released from stressed or dying cells activate dendritic cells (DCs), transforming them into mature APCs. Hsp endocytosis by DCs increases the cell surface expression of MHC class II molecules, in addition to several costimulatory molecules, thereby potentiating immune recognition of antigens [27]. Mature DCs can program lymphocyte effector cells in an antigen-restricted manner, thus limiting collateral damage to normal healthy tissues, which do not express the target antigen. The ability of Hsps to chaperone proteins prior to endocytosis and processing by DCs can potentially broaden the repertoire of presented epitopes and thus, the spectrum of the immune response [28].
Germane to this review, tumor cells express unique proteins that may serve as antigens for targeted immune responses. The significant number of mutations present in any tumor cell can create protein products with unique antigens and epitopes for recognition by the immune system as foreign bodies. Tumor cells also possess abundant amounts of constitutively expressed Hsps [24, 29]. In theory, fever-range hyperthermia may take advantage of tumor cell Hsps by inducing their release from tumor cells and augmenting DC priming against tumor antigens. In several models of hyperthermia, heat-treated tumors exhibited improved DC priming and generation of systemic immunity to tumor cells [30–32], which, in one study, could be abrogated by the loss of Hsp70 expression [30]. It has also been reported that hyperthermia alone can enhance antigen display by tumor cells, thus rendering them even more susceptible to programmed immune clearance [33, 34]. Fever-range hyperthermia may also induce Hsps [35], and has been found to improve the activity of Hsp-based vaccine strategies [36], an approach further discussed in the following section.
Hsps as cancer vaccines
Ongoing research efforts are evaluating the potential role of Hsp-peptide complexes isolated from cancer cells, as well as Hsps complexed to tumor antigens, as cancer vaccine candidates. Comprehensive and detailed reviews have been published on this subject [37–43] and should supplement the outline this topic that follows. A major potential benefit of utilizing Hsps in vaccine development is that a dominant tumor antigen may not have to be fully characterized or isolated to be complexed to the Hsp of interest[44], which is unique to only a few other vaccine strategies [45, 46]. Using tumor cell lysates as a rich source of Hsp-peptide complexes, several tumor antigens could be presented to APCs, including multiple epitopes of the same antigen. The use of Hsps may exert an adjuvant effect by bolstering MHC class II and co-stimulatory molecule expression by DCs [47].
The ultimate goal of using Hsps in cancer vaccine cliniclatrials is to assess whether the published data derived from animal models of disease, demonstrating the generation of effective antitumor responses in their presence, can be replicated in humans [44, 48, 49]. Extensive knowledge obtained from phase I and phase II clinical studies using autologous, tumor-derived Hsp (gp96)-peptide complex (vitespen, Antigenics Inc) for the treatment of melanoma, colorectal, renal cell and pancreatic carcinoma [50–53], lead to a phase III clinical trial aiming to determine the clinical efficacy of the vaccine. This phase III trial for renal cell carcinoma suggested a potential benefit of the vaccine for patients with early-stage disease, but did not demonstrate statistically significant differences in recurrence-free survival as a clinical endpoint [52, 54]. The ability to isolate and administer an Hsp-peptide complex from patient tumors, as demonstrated by this study, offers the hope of individualized therapeutic treatment for cancer patients based upon promising preclinical data.
In theory, an autologous, tumor-derived Hsp-peptide vaccine can be generated by applying fever-range hyperthermia or thermal ablation to tumor sites without the need for ex vivo manipulations, but this technique has yet to be systematically evaluated. For example, various histological findings associated with thermal ablation of tumors have been described, including thermal fixation and narrow reactive zones [55]. Thermal fixation implies preservation of cellular architecture with resistance to breakdown and a lack of wound healing responses. Inflammatory reactive zones, however, may create an ‘interface’ between tumor antigens and the immune system mediated by the release of Hsps [56]. The thermal ablation of liver tumors in particular has demonstrated an ability to potentiate immune responses [57, 58] and elicit robust T-cell infiltrates at ablation sites [59, 60]. Similar to thermal ablation methods, fever-range hyperthermia may foster interactions between tumors and the immune system and is an area of active research. More specifically, the ability of fever-range hyperthermia to induce reactive immunity against tumor antigens through DCs and NK-cells is likely mediated by Hsps, and is discussed in the following sections.
Improvement of dendritic cell and NK-cell function by hyperthermia
Hsps and dendritic cell activation
The release of Hsps from tumor cells can serve as a potent activating signal for quiescent APCs. Accordingly, the ability to induce DC maturation seems directly proportional to the Hsp content of tumor cells [29]. Moreover, the ability of tumor cell lysates from a murine thymoma to induce maturation of DCs could be experimentally abrogated by the use of the Hsp90 inhibitor geldanamycin [29]. Additionally, HSPs are able to mediate the cross-priming of tumor antigens [28, 61, 62]. Cross-priming is the ability of extracellular Hsps complexed to tumor peptides to be internalized and presented in the context of MHC class I molecules on APCs, thus allowing potent priming of CTLs against tumor antigens. It has been reported that Hsps are generated from necrotic tumor cell lysates, but not from tumor cells undergoing apoptosis [63–65]. There are also published data demonstrating that necrotic tumor cell lysates enhance antigen cross-presentation more efficiently compared with early apoptotic tumor cells [66]. In contrast, there are conflicting data demonstrating improved DC activation with apoptotic renal, squamous and pancreatic carcinoma cells, suggesting possible differences between cancer cell types and/or cell lines [67–69]. These differences in tumor features are not trivial, as hyperthermia may serve as a mediator of either tumor cell necrosis or apoptosis depending on the temperature used and the exposure time. Temperature, time and other factors associated with hyperthermia and cell killing have been comprehensively reviewed [70], and are likely to determine the mode of cell death. In tumor cells exposed to hyperthermia in the heat shock range (42°C for 4h) prior to lysing, DC activation and cross-priming were significantly enhanced with the application of heat [33]. Enhanced cross-priming was directly attributed to increased expression of Hsps in hyperthermia-treated cells [33].
Hyperthermia and dendritic cell-based cancer vaccines
The clinical use of tumor-primed DC vaccines can be hampered by several factors, including the availability of usable tumor tissue, dependence upon ex vivo and in vivo antigen loading and a potential lack of appropriate recruitment of DCs. In an effort to overcome these barriers, a preclinical study evaluated the use of hyperthermia combined with intratumoral injection of DCs to treat melanoma [71]. This study aimed to assess the proposed benefits of in situ tumor-antigen loading of DCs in the presence of local stimulating factors elicited by hyperthermia. A significant inhibition of tumor growth was noted, with concomitant migration of injected DCs to tumor draining lymph nodes and priming of CTLs [71]. Similar findings were obtained in a small clinical study using this treatment strategy [72]. Patients with advanced melanoma were treated with intratumoral injections of immature DCs with or without adjuvant local hyperthermia. The addition of hyperthermia extended the time to tumor progression, improved T-lymphocyte priming, and created a favorable balance between effector and regulatory T cell (Treg) tumor infiltration [72].
Due to the ability of Hsps to activate DCs directly by chaperoning tumor antigens upon their release [28], it is possible that both local and regional immune stimulation can be achieved with hyperthermia. In a recent study using a murine prostate cancer model, localized hyperthermia was combined with intratumoral injection of DCs [73]. Intratumoral DCs engulfed tumor cell fragments generated by hyperthermia and induced a systemic T-cell-mediated immune response. Most importantly, tumors treated with a combination of hyperthermia and intratumoral DC injections exhibited significant growth inhibition compared with controls. In this study, hyperthermia was reported to generate both necrotic and apoptotic tumor cells, which may reflect the clinical realities of treating heterogeneous tumor tissues [73]. In another study using a murine lung carcinoma model, Hsps released following local heat shock (42–43°C) treatment of tumors acted as potent autocrine and paracrine signaling molecules [30]. Tumor cells exposed to the released Hsps produced chemoattractants (like CCL2 {chemokine [C-C motif] ligand 2}, CCL5 and CXCL10 {chemokine [C-X-C motif] ligand 10}) that improved migration of DCs in vitro and was dependent upon TLR4 expression on tumor cells. Interestingly, DCs in these experiments were directly activated by tumor-derived Hsp70 via a TLR4-dependent mechanism present in the DCs [30]. Collectively, these findings support the use of hyperthermia as an inducer of Hsps to serve as ‘danger signals’, activating antitumor immune responses.
Attempts at targeting endogenous DCs to specific anatomic sites have also been evaluated by Hsp-tumor antigen complex injection [74]. The activation of anatomically distinct DCs could be manipulated by varying the mode of Hsp-tumor antigen injection (ie, intravenous versus subcutaneous). Moreover, the Hsp-tumor antigen complex could be conjugated to specific molecules, such as polyhistidine, to enhance intracellular trafficking upon endocytosis by DCs, thus strengthening the priming of CTLs [74]. Efficient intracellular trafficking could obviate the necessity for direct intratumora linjection, while maintaining the vaccination efficacy of DCs.
Hyperthermia and immune tolerance
Tumor-induced immune tolerance continues to represent a significant barrier to effective cancer immunotherapies. Tregs contribute to immune tolerance [75], but their behavior has not been extensively evaluated in the context of hyperthermia. Immature myeloid cells (iMCs) derived from the bone marrow of tumor-bearing hosts have been identified as significant suppressors of antitumor immune responses [76, 77]. Derived from iMC precursors, DCs function as key regulators of T-cell activity [78]. Differentiation of a subset of iMCs to mature DCs is inhibited by several postulated tumor-derived factors [76, 77]. However, differentiation of DCs to mature cells capable of presenting tumor antigens may be elicited through the application of hyperthermia [79, 80]. Whether hyperthermia can overcome tumor-induced immune tolerance and induce differentiation of iMCs has yet to be determined experimentally. The use of hyperthermia applied to DCs recovered from patients with medullary thyroid cancer was examined in a preliminary clinical study [81]. Hyperthermia-treated DCs enibited enhanced priming capacity and were more potent stimulators CTLs compared with controls. An analogous clinical study investigating the effects of hyperthermia in human hepatocellular carcinoma reported similar results [32]. Moreover, it should be noted that whole-body hyperthermia not only augments immune responses, but also stimulates the migration of skin-derived DCs to draining lymph nodes [82]. These preclinical findings suggest a valuable role of hyperthermia in DC cancer vaccine strategies.
Hyperthermia and NK cells
As an important mediator of innate antitumor immunity, NK-cells have been reported to be responsive to hyperthermia [83–85]. The importance of NK cells in mediating antitumor activity during hyperthermia treatment was demonstrated in mice bearing either human breast tumor xenografts or syngeneic tumors [86]. In mice treated with fever-range whole-body hyperthermia, tumor growth was significantly inhibited and NK-cell infiltration increased compared with control-treated animals. In this study, NK cells were required for the antitumor effects observed [86]. The mechanisms involved in hyperthermia-mediated activation of NK cells were addressed in a separate murine study [87]. Although an increase in the cell-surface expression of activating NK-cell receptors was not noted, clustering of these activating receptors appeared to be induced by the hyperthermia. The clustering of receptors in addition to increased tumor surface expression of an NK activating ligand, MICA (MHC-class-I-chain-related gene A), was postulated to improve NK-mediated antitumor activities [87].
In clinical trials, exposure to fever-range hyperthermia resulted in improved endogenous NK-cell cytotoxicity to several cancer types [88, 89]. The distribution of NK cells throughout the body could also be altered by whole-body hyperthermia, with increased numbers observed in peripheral blood samples, suggesting mobili9zation and the possibility for improved immunosurveillance [90, 91].
Collectively, the evidence for improved activation and function of DCs and NK cells following hyperthermia treatment justifies further investigation within clinical trials of the effect of hyperthermia on these cell types in the oncology setting.
Enhancement of immunotherapies by hyperthermia
Improved lymphocyte-endothelial adhesion and leukocyte trafficking
Leukocyte trafficking is a highly regulated and orchestrated process that is tightly regulated by hyperthermia at multiple levels [92, 93]. Whole-body hyperthermia in the fever range has been demonstrated to regulate adhesion molecule expression on select vascular endothelial sites [92–94]. Hyperthermia increases the expression ICAM-1 a key adhesion molecule, on high endothelial venules of secondary lymphoid tissues. Lymph node high endothelial venules and Peyer’s patches are efficient portals of lymphocyte entry into areas of interaction with APCs such as DCs [95]. Hyperthermia can augment this already efficient system and allow for increased lymphocyte entry across this specialized endothelial layer [93].
Remarkably, hyperthermia can also act directly on lymphocytes to improve their adhesive properties [94, 96–98]. Lymphocytes exposed to fever-range hyperthermia demonstrated enhanced L-selectin/α4β7 integrin affinity and/or avidity for endothelial adhesion molecules, ultimately leading to improved homing to lymphoid tissues compared with nornothermal controls. The combined effects of hyperthermia on lymphoid tissue endothelium and lymphocytes can promote immune surveillance and increase the probability of naive lymphocytes leaving the circulation and encountering their cognate antigen displayed by DCs in lymphoid organs. In independent clinical studies, whole-body hyperthermia resulted in a transient decrease in circulating lymphocytes in patients with advanced cancer [12, 94, 99, 100], a finding which mirrored observations in animal models in which lymphocyte entry into lymph noeds was increased following hyperthermia treatment [93]. Enhanced recruitment of lymphocytes to lymphoid tissues may be exploited in the treatment of malignancies.
There is further evidence demonstrating that hyperthermia can enhance lymphocyte-endothelial interactions by regulating a specific class of chemoattractant molecules. Chemokines are chemoattractant molecules that control leukocyte trafficking and migration and are responsive to hyperthermic stimuli [101]. Fever-range, whole-body hyperthermia strongly increases the intravascular display of CCL21, a key homeostatic chemokine, which mediates lymphocyte trafficking across high endothelial venules [93]. Moreover, certain inflammatory chemokines (IL-8, as well as other CXC chemokines) have been proposed to be classical Hsps based on their regulation by Hsp transcription factors [101, 102]. It has been hypothesized that evolutionary changes have intertwined heat shock responses with enhanced chemoattraction as would be required for neutrophil recruitment to sites of infection during febrile illnesses [101].
The emerging picture from these studies is that hyperthermia acts at multiple levels to improve lymphocyte and endothelial interactions via complex complementary mechanisms involving a variety of trafficking molecules. Further studies are required to determine whether hyperthermia can be exploited in immunotherapy protocols to enhance trafficking of immune effector cells to tumor sites. Support for such studies is provided by the finding that there is increase accumulation of endogenous neutrophils and lymphocytes in the tumor microenvironment following exposure to hyperthermia. More specifically, in inflammatory models, fever-range hyperthermia has been found to generate neutrophil accumulation at sites of infection, as well as at tumor sites [86, 103–105]. Increased leukocyte migration in inflammatory models appears to be correlated to chemoattractants elicited by exposure to hyperthermia. The synergy between local Hsp peptide release and chemokine production may serve to amplify immune responses at tumor sites exposed to hyperthermia. Preliminary preclinical and clinical studies suggest there is a benefit to the addition of hyperthermia to adoptive immunotherapy protocols, which may be related to trafficking mechanisms [106]. The ability of fever-range hyperthermia to enhance adoptively transferred lymphocyte homing to tumors is an area of active exploration.
Hyperthermia-mediated immune surveillance in cancer
The circulation of lymphocytes through lymphoid tissues may be mandatory for the generation of cancer immunity. Endogenous immune responses to tumors occur within the unique environment of draining lymph nodes [107]. The initial tumor antigen presentation and initiation of clonal expansion of CTLs transpires in the lymph nodes and cannot take place outside this specialized compartment [107]. Furthermore, the ability of DCs present in the lymph nodes to stimulate an anti-tumor immune response is critical [108]. As hyperthermia has been shown to improve immune surveillance by T-cells [93] and to increase DC trafficking to lymph nodes [82], the application of fever-range, whole-body hyperthermia merits further investigation in cancer immunology protocols.
In addition, the ‘seeding’ of lymphoid tissue by exogenous, adoptively transferred antitumor lymphocytes may prove to have profound clinical significance [109]. Accordingly, the depletion of host lymphocytes prior to adoptive immunotherapy appears important for enhanced therapeutic efficacy. In immunodepleted hosts, there is less competition for cytokines that may promote the effector function of adoptively transferred antitumor lymphocytes. The process of immunodepletion with a combination of low-dose chemotherapy and non-lethal total-body radiation may also eliminate any endogenous immune suppressor-cell populations that can dampen the efficacy of adoptive transfers. One proposed mechanism of enhanced efficacy is the homeostatic expansion of adoptively transferred lymphocytes in ‘empty’ lymphoid compartments [110]. Further activation and expansion of adoptively transferred CTLs in lymph nodes may also be crucial for improving antitumor responses, as demonstrated by the utilization of minimally cultured tumor-infiltrating lymphocytes [111]. Increased costimulation of less differentiated, adoptively transferred lymphocytes by endogenous DCs may act to amplify antitumor effects. Hyperthermia may supplement these requirements and represent a useful adjunct to adoptive transfer regimens by increasing the entry of adoptively transferred lymphocytes into lymphoid compartments. Fever-range hyperthermia is being evaluated in preclinical studies regarding these issues.
Conclusion
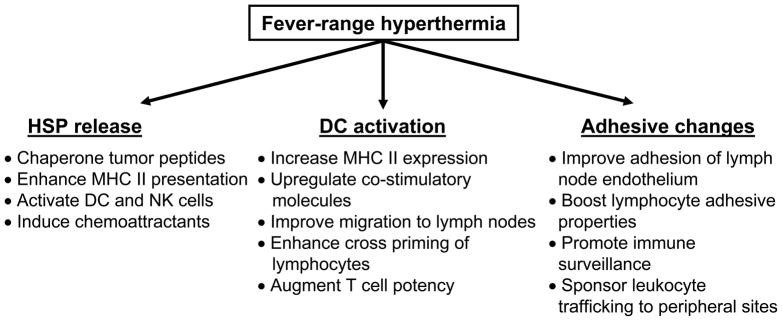
Insight into the mechanisms of hyperthermia and the influence of Hsps on the immune system has created a cornerstone for future investigations on their potential use in cancer treatment. A new emphasis on lymphocyte trafficking to lymphoid tissues, programming by DCs and chemoattraction to tumor sites may be supported by the pleiotropic effects of hyperthermia, as summarized in Figure 2. The use of hyperthermia as an adjuvant to existing immune therapy regimens represents a non-toxic, readily achievable treatment modality that has the potential to reinvigorate marginally efficacious protocols for the treatment of cancer.
Figure 2. Antitumor effects of fever-range hyperthermia.
Beneficial immune consequences of fever-range hyperthermia are mediated via Hsp peptide release, dendritic cell (DC) activation and changes in trafficking, including cellular adhesive and immune surveillance
Acknowledgments
The author would like to recognize the outstanding contributions to this field by all members of the Sharon Evans, Elizabeth Repasky and John Subjeck research laboratories, who continually define excellence in research.
References
•• of outstanding interest
• of special interest
- 1 •.Mackowiak PA. Concepts of fever. Arch Intern Med. 1998(158):17–1870. doi: 10.1001/archinte.158.17.1870. Evaluates the history of fever in medicine and the many benefits conferred bythe process defined as fever. [DOI] [PubMed] [Google Scholar]
- 2 ••.Dewhirst MW, et al. Re-setting the biologic rationale for thermal therapy. Int J Hyperthermia. 2005(21):8–779. doi: 10.1080/02656730500271668. Reviews the history of clinical and experimental hyperthermia and defines the basis for future clinical investigations. [DOI] [PubMed] [Google Scholar]
- 3.Tonnesen AS, et al. Sweating, hemodynamic responses, and thermal equilibration during hyperthermia in humans. J Appl Physiol. 1987;62(4):1596–602. doi: 10.1152/jappl.1987.62.4.1596. [DOI] [PubMed] [Google Scholar]
- 4.Hall EJ, Roizin-Towle L. Biological effects of heat. Cancer Res. 1984;44(10 Suppl):4708s–4713s. [PubMed] [Google Scholar]
- 5.Moyer HR, Delman KA. The role of hyperthermia in optimizing tumor response to regional therapy. Int J Hyperthermia. 2008;24(3):251–61. doi: 10.1080/02656730701772480. [DOI] [PubMed] [Google Scholar]
- 6.Nagata Y, et al. Clinical results of radiofrequency hyperthermia for malignant liver tumors. Int J Radiat Oncol Biol Phys. 1997;38(2):359–65. doi: 10.1016/s0360-3016(96)00625-6. [DOI] [PubMed] [Google Scholar]
- 7.Wondergem J, et al. Effect of adriamycin combined with whole body hyperthermia on tumor and normal tissues. Cancer Res. 1991;51(13):3559–67. [PubMed] [Google Scholar]
- 8.Calderwood SK, Theriault JR, Gong J. How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia. 2005;21(8):713–6. doi: 10.1080/02656730500340794. [DOI] [PubMed] [Google Scholar]
- 9.Turtle CJ, Hart DN. Dendritic cells in tumor immunology and immunotherapy. Curr Drug Targets. 2004;5(1):17–39. doi: 10.2174/1389450043490640. [DOI] [PubMed] [Google Scholar]
- 10.Mackowiak PA, Worden G. Carl Reinhold August Wunderlich and the evolution of clinical thermometry. Clin Infect Dis. 1994;18(3):458–67. doi: 10.1093/clinids/18.3.458. [DOI] [PubMed] [Google Scholar]
- 11.Dewhirst MW, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19(3):267–94. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 12.Kraybill WG, et al. A phase I study of fever-range whole body hyperthermia (FR-WBH) in patients with advanced solid tumours: correlation with mouse models. Int J Hyperthermia. 2002;18(3):253–66. doi: 10.1080/02656730110116704. [DOI] [PubMed] [Google Scholar]
- 13.Ritossa F. A new puffing patern induced by temperature shock and DNP in Drosophila. Experientia. 1962;18:571–573. [Google Scholar]
- 14.Liberek K, Lewandowska A, Zietkiewicz S. Chaperones in control of protein disaggregation. Embo J. 2008;27(2):328–35. doi: 10.1038/sj.emboj.7601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24(1):31–9. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- 16.Connell P, et al. The co-chaperone CHIP regulates protein triage decisions mediated by heat-shock proteins. Nat Cell Biol. 2001;3(1):93–6. doi: 10.1038/35050618. [DOI] [PubMed] [Google Scholar]
- 17.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228(2):111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 18.Aghdassi A, et al. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67(2):616–25. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 19.Jones EL, et al. The 70 kilodalton heat shock protein is an inhibitor of apoptosis in prostate cancer. Int J Hyperthermia. 2004;20(8):835–49. doi: 10.1080/02656730410001721807. [DOI] [PubMed] [Google Scholar]
- 20.Xiang TX, et al. RNA interference-mediated silencing of the Hsp70 gene inhibits human gastric cancer cell growth and induces apoptosis in vitro and in vivo. Tumori. 2008;94(4):539–50. doi: 10.1177/030089160809400416. [DOI] [PubMed] [Google Scholar]
- 21.Li GC, Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982;79(10):3218–22. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohnishi K, et al. Effects of a heat shock protein inhibitor KNK437 on heat sensitivity and heat tolerance in human squamous cell carcinoma cell lines differing in p53 status. Int J Radiat Biol. 2004;80(8):607–14. doi: 10.1080/09553000412331283470. [DOI] [PubMed] [Google Scholar]
- 23.Ehrnsperger M, et al. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. Embo J. 1997;16(2):221–9. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24 ••.Calderwood SK, et al. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006(31):3–164. doi: 10.1016/j.tibs.2006.01.006. Defines the role of specific Hsps in a variety of cancer cell processes, demonstrating the ubiquitous nature of Hsps in supporting the malignant phenotype. [DOI] [PubMed] [Google Scholar]
- 25.Tamura Y, et al. 70 kDa heat shock cognate protein is a transformation-associated antigen and a possible target for the host’s anti-tumor immunity. J Immunol. 1993;151(10):5516–24. [PubMed] [Google Scholar]
- 26.Todryk SM, et al. Heat shock proteins refine the danger theory. Immunology. 2000;99 (3):334–7. doi: 10.1046/j.1365-2567.2000.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 •.Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. Reviews the extensive role of Hsps in the modulation of a diverse number of immune responses. [DOI] [PubMed] [Google Scholar]
- 28.Bendz H, et al. Human heat shock protein 70 enhances tumor antigen presentation through complex formation and intracellular antigen delivery without innate immune signaling. J Biol Chem. 2007;282(43):31688–702. doi: 10.1074/jbc.M704129200. [DOI] [PubMed] [Google Scholar]
- 29 •.Somersan S, et al. Primary tumor tissue lysates are enriched in heat shock proteins and induce the maturation of human dendritic cells. J Immunol. 2001(167):9–4844. doi: 10.4049/jimmunol.167.9.4844. Describes a preclinical study that provided evidence supporting the physiological relevance of Hsp-rich tumor lysates in inducing DC activation, thereby establishing a potential role for Hsps in cancer imunotherapy. [DOI] [PubMed] [Google Scholar]
- 30.Chen T, et al. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182(3):1449–59. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 31.Bleifuss E, et al. Differential capacity of chaperone-rich lysates in cross-presenting human endogenous and exogenous melanoma differentiation antigens. Int J Hyperthermia. 2008;24(8):623–37. doi: 10.1080/02656730802213384. [DOI] [PubMed] [Google Scholar]
- 32.Schueller G, et al. Hyperthermia improves cellular immune response to human hepatocellular carcinoma subsequent to co-culture with tumor lysate pulsed dendritic cells. Int J Oncol. 2003;22(6):1397–402. [PubMed] [Google Scholar]
- 33.Shi H, et al. Hyperthermia enhances CTL cross-priming. J Immunol. 2006;176(4):2134–41. doi: 10.4049/jimmunol.176.4.2134. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T, et al. Modifications of tumor-associated antigen expression on human lung cancer cells by hyperthermia and cytokine. Anticancer Res. 1995;15(6B):2601–6. [PubMed] [Google Scholar]
- 35.Di YP, Repasky EA, Subjeck JR. Distribution of HSP70, protein kinase C, and spectrin is altered in lymphocytes during a fever-like hyperthermia exposure. J Cell Physiol. 1997;172(1):44–54. doi: 10.1002/(SICI)1097-4652(199707)172:1<44::AID-JCP5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 36.Wang XY, et al. Characterization of heat shock protein 110 and glucose-regulated protein 170 as cancer vaccines and the effect of fever-range hyperthermia on vaccine activity. J Immunol. 2001;166(1):490–7. doi: 10.4049/jimmunol.166.1.490. [DOI] [PubMed] [Google Scholar]
- 37.Murshid A, Gong J, Calderwood SK. Heat-shock proteins in cancer vaccines: agents of antigen cross-presentation. Expert Rev Vaccines. 2008;7(7):1019–30. doi: 10.1586/14760584.7.7.1019. [DOI] [PubMed] [Google Scholar]
- 38 •.Srivastava PK. Immunotherapy for human cancer using heat shock protein-peptide complexes. Curr Oncol Rep. 2005(7):2–104. doi: 10.1007/s11912-005-0035-8. A leading authority in Hsps reviews the rationale for Hsp-based immunotherapy of tumors. [DOI] [PubMed] [Google Scholar]
- 39.Oki Y, Younes A. Heat shock protein-based cancer vaccines. Expert Rev Vaccines. 2004;3(4):403–11. doi: 10.1586/14760584.3.4.403. [DOI] [PubMed] [Google Scholar]
- 40.Wang XY, et al. Targeted immunotherapy using reconstituted chaperone complexes of heat shock protein 110 and melanoma-associated antigen gp100. Cancer Res. 2003;63 (10):2553–60. [PubMed] [Google Scholar]
- 41.Manjili MH, et al. Development of a recombinant HSP110-HER-2/neu vaccine using the chaperoning properties of HSP110. Cancer Res. 2002;62(6):1737–42. [PubMed] [Google Scholar]
- 42.Wang XY, et al. Heat shock proteins and cancer immunotherapy. Immunol Invest. 2000;29 (2):131–7. doi: 10.3109/08820130009062296. [DOI] [PubMed] [Google Scholar]
- 43.Pardoll DM. Cancer vaccines. Nat Med. 1998;4(5 Suppl):525–31. doi: 10.1038/nm0598supp-525. [DOI] [PubMed] [Google Scholar]
- 44.Tamura Y, et al. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278(5335):117–20. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 45.de Gruijl TD, et al. Whole-cell cancer vaccination: from autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother. 2008;57 (10):1569–77. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shu S, et al. Immunogenicity of dendritic-tumor fusion hybrids and their utility in cancer immunotherapy. Crit Rev Immunol. 2007;27(5):463–83. doi: 10.1615/critrevimmunol.v27.i5.50. [DOI] [PubMed] [Google Scholar]
- 47.Binder RJ, et al. Cutting edge: heat shock protein gp96 induces maturation and migration of CD11c+ cells in vivo. J Immunol. 2000;165(11):6029–35. doi: 10.4049/jimmunol.165.11.6029. [DOI] [PubMed] [Google Scholar]
- 48.Kim HL, et al. Evaluation of renal cell carcinoma vaccines targeting carbonic anhydrase IX using heat shock protein 110. Cancer Immunol Immunother. 2007;56 (7):1097–105. doi: 10.1007/s00262-006-0258-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang XY, et al. Extracellular targeting of endoplasmic reticulum chaperone glucose-regulated protein 170 enhances tumor immunity to a poorly immunogenic melanoma. J Immunol. 2006;177(3):1543–51. doi: 10.4049/jimmunol.177.3.1543. [DOI] [PubMed] [Google Scholar]
- 50.Belli F, et al. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J Clin Oncol. 2002;20(20):4169–80. doi: 10.1200/JCO.2002.09.134. [DOI] [PubMed] [Google Scholar]
- 51.Mazzaferro V, et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9 (9):3235–45. [PubMed] [Google Scholar]
- 52.Wood C, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. Lancet. 2008;372(9633):145–54. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 53.Maki RG, et al. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig Dis Sci. 2007;52(8):1964–72. doi: 10.1007/s10620-006-9205-2. [DOI] [PubMed] [Google Scholar]
- 54.Jonasch E, et al. Vaccination of metastatic renal cell carcinoma patients with autologous tumour-derived vitespen vaccine: clinical findings. Br J Cancer. 2008;98 (8):1336–41. doi: 10.1038/sj.bjc.6604266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coad JE, et al. Radiofrequency ablation causes ‘thermal fixation’ of hepatocellular carcinoma: a post-liver transplant histopathologic study. Clin Transplant. 2003;17 (4):377–84. doi: 10.1034/j.1399-0012.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia. 2007;23(2):165–71. doi: 10.1080/02656730701206638. [DOI] [PubMed] [Google Scholar]
- 57.Napoletano C, et al. RFA strongly modulates the immune system and anti-tumor immune responses in metastatic liver patients. Int J Oncol. 2008;32(2):481–90. [PubMed] [Google Scholar]
- 58.Zerbini A, et al. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66 (2):1139–46. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]
- 59.Hansler J, et al. Cellular and vascular reactions in the liver to radio-frequency thermo-ablation with wet needle applicators. Study on juvenile domestic pigs. Eur Surg Res. 2002;34(5):357–63. doi: 10.1159/000064000. [DOI] [PubMed] [Google Scholar]
- 60.Wissniowski TT, et al. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63(19):6496–500. [PubMed] [Google Scholar]
- 61.Kurotaki T, et al. Efficient cross-presentation by heat shock protein 90-peptide complex-loaded dendritic cells via an endosomal pathway. J Immunol. 2007;179(3):1803–13. doi: 10.4049/jimmunol.179.3.1803. [DOI] [PubMed] [Google Scholar]
- 62.Srivastava PK, et al. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39(2):93–8. doi: 10.1007/BF00188611. [DOI] [PubMed] [Google Scholar]
- 63.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191(3):423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu S, et al. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12(11):1539–46. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 65.Berwin B, Reed RC, Nicchitta CV. Virally induced lytic cell death elicits the release of immunogenic GRP94/gp96. J Biol Chem. 2001;276(24):21083–8. doi: 10.1074/jbc.M101836200. [DOI] [PubMed] [Google Scholar]
- 66.Brusa D, et al. Post-apoptotic tumors are more palatable to dendritic cells and enhance their antigen cross-presentation activity. Vaccine. 2008;26(50):6422–32. doi: 10.1016/j.vaccine.2008.08.063. [DOI] [PubMed] [Google Scholar]
- 67.D’ Hooghe E, et al. Apoptic renal carcinoma cells are better inducers of cross-presenting activity than their primary necrotic counterpart. Int J Immunopathol Pharmacol. 2007;20(4):707–17. doi: 10.1177/039463200702000406. [DOI] [PubMed] [Google Scholar]
- 68.Hoffmann TK, et al. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 2000;60(13):3542–9. [PubMed] [Google Scholar]
- 69.Schnurr M, et al. Apoptotic pancreatic tumor cells are superior to cell lysates in promoting cross-priming of cytotoxic T cells and activate NK and gammadelta T cells. Cancer Res. 2002;62(8):2347–52. [PubMed] [Google Scholar]
- 70 •.Roti Roti JL. Cellular responses to hyperthermia (40–46 degrees C): cell killing and molecular events. Int J Hyperthermia. 2008(24):1–3. doi: 10.1080/02656730701769841. Provides a succinct summary of the effects of hyperthermia on cellular processes, with a focus on the kinetics and mechanisms involved. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka K, et al. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer. 2005;116(4):624–33. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 72.Guo J, et al. Intratumoral injection of dendritic cells in combination with local hyperthermia induces systemic antitumor effect in patients with advanced melanoma. Int J Cancer. 2007;120(11):2418–25. doi: 10.1002/ijc.22551. [DOI] [PubMed] [Google Scholar]
- 73.Mukhopadhaya A, et al. Localized hyperthermia combined with intratumoral dendritic cells induces systemic antitumor immunity. Cancer Res. 2007;67(16):7798–806. doi: 10.1158/0008-5472.CAN-07-0203. [DOI] [PubMed] [Google Scholar]
- 74.Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354(1–2):23–7. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- 75.Qin FX. Dynamic behavior and function of Foxp3+ regulatory T cells in tumor bearing host. Cell Mol Immunol. 2009;6(1):3–13. doi: 10.1038/cmi.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76 •.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006(55):3–237. doi: 10.1007/s00262-005-0048-z. Examines the role of iMCs as inhibitors of antitumor immune responses, thereby defining another potential barrier to effective immunotherapies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim R, Emi M, Tanabe K. Cancer immunosuppression and autoimmune disease: beyond immunosuppressive networks for tumour immunity. Immunology. 2006;119(2):254–64. doi: 10.1111/j.1365-2567.2006.02430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caux C, et al. Dendritic cell biology and regulation of dendritic cell trafficking by chemokines. Springer Semin Immunopathol. 2000;22(4):345–69. doi: 10.1007/s002810000053. [DOI] [PubMed] [Google Scholar]
- 79.Basu S, Srivastava PK. Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int Immunol. 2003;15(9):1053–61. doi: 10.1093/intimm/dxg104. [DOI] [PubMed] [Google Scholar]
- 80.Ostberg JR, Repasky EA. Emerging evidence indicates that physiologically relevant thermal stress regulates dendritic cell function. Cancer Immunol Immunother. 2006;55 (3):292–8. doi: 10.1007/s00262-005-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bachleitner-Hofmann T, et al. Heat shock treatment of tumor lysate-pulsed dendritic cells enhances their capacity to elicit antitumor T cell responses against medullary thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(11):4571–7. doi: 10.1210/jc.2006-0971. [DOI] [PubMed] [Google Scholar]
- 82.Ostberg JR, et al. Regulatory potential of fever-range whole body hyperthermia on Langerhans cells and lymphocytes in an antigen-dependent cellular immune response. J Immunol. 2001;167(5):2666–70. doi: 10.4049/jimmunol.167.5.2666. [DOI] [PubMed] [Google Scholar]
- 83.Dayanc BE, et al. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia. 2008;24(1):41–56. doi: 10.1080/02656730701858297. [DOI] [PubMed] [Google Scholar]
- 84.Kappel M, et al. Effects of in vivo hyperthermia on natural killer cell activity, in vitro proliferative responses and blood mononuclear cell subpopulations. Clin Exp Immunol. 1991;84(1):175–80. doi: 10.1111/j.1365-2249.1991.tb08144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Downing JF, Taylor MW. The effect of in vivo hyperthermia on selected lymphokines in man. Lymphokine Res. 1987;6(2):103–9. [PubMed] [Google Scholar]
- 86.Burd R, et al. Tumor cell apoptosis, lymphocyte recruitment and tumor vascular changes are induced by low temperature, long duration (fever-like) whole body hyperthermia. J Cell Physiol. 1998;177(1):137–47. doi: 10.1002/(SICI)1097-4652(199810)177:1<137::AID-JCP15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 87.Ostberg JR, et al. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J Leukoc Biol. 2007 doi: 10.1189/jlb.1106699. [DOI] [PubMed] [Google Scholar]
- 88.Zanker KS, Lange J. Whole body hyperthermia and natural killer cell activity. Lancet. 1982;1(8280):1079–80. doi: 10.1016/s0140-6736(82)92142-0. [DOI] [PubMed] [Google Scholar]
- 89.Nakayama J, et al. Kinetics of immunological parameters in patients with malignant melanoma treated with hyperthermic isolated limb perfusion. J Dermatol Sci. 1997;15 (1):1–8. doi: 10.1016/s0923-1811(96)00587-7. [DOI] [PubMed] [Google Scholar]
- 90.Ahlers O, et al. Stress induced changes in lymphocyte subpopulations and associated cytokines during whole body hyperthermia of 41.8–42.2 degrees C. Eur J Appl Physiol. 2005;95(4):298–306. doi: 10.1007/s00421-005-0009-4. [DOI] [PubMed] [Google Scholar]
- 91.Blazickova S, et al. Effect of hyperthermic water bath on parameters of cellular immunity. Int J Clin Pharmacol Res. 2000;20(1–2):41–6. [PubMed] [Google Scholar]
- 92.Chen Q, et al. Thermal facilitation of lymphocyte trafficking involves temporal induction of intravascular ICAM-1. Microcirculation. 2009;16(2):143–58. doi: 10.1080/10739680802353850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93 ••.Chen Q, et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat Immunol. 2006;7(12):1299–1308. doi: 10.1038/ni1406. Discusses the role of hyperthermia in improving immune surveillance, and at the same time defines the contributing mechanisms associated with improved endothelial adhesion. [DOI] [PubMed] [Google Scholar]
- 94.Evans SS, et al. Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood. 2001;97(9):2727–33. doi: 10.1182/blood.v97.9.2727. [DOI] [PubMed] [Google Scholar]
- 95.Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16(9):449–57. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- 96 •.Chen Q, et al. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity. 2004;20(1):59–70. doi: 10.1016/s1074-7613(03)00358-3. Describes the molecular mechanisms associates with improved lymphocytes ashesion following fever-range hyperthermia. [DOI] [PubMed] [Google Scholar]
- 97.Evans SS, et al. Dynamic association of L-selectin with the lymphocyte cytoskeletal matrix. J Immunol. 1999;162(6):3615–24. [PubMed] [Google Scholar]
- 98.Wang WC, et al. Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol. 1998;160(2):961–9. [PubMed] [Google Scholar]
- 99.Ostberg JR, Repasky EA. Comparison of the effects of two different whole body hyperthermia protocols on the distribution of murine leukocyte populations. Int J Hyperthermia. 2000;16(1):29–43. doi: 10.1080/026567300285402. [DOI] [PubMed] [Google Scholar]
- 100.Atanackovic D, et al. Patients with solid tumors treated with high-temperature whole body hyperthermia show a redistribution of naive/memory T-cell subtypes. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R585–94. doi: 10.1152/ajpregu.00014.2005. [DOI] [PubMed] [Google Scholar]
- 101 •.Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: a new family of heat-shock proteins? Immunol Invest. 2005(34):3–381. doi: 10.1081/imm-200067648. Examines chemokines in the context of Hsp-related promoters. [DOI] [PubMed] [Google Scholar]
- 102.Singh IS, et al. Heat shock co-activates interleukin-8 transcription. Am J Respir Cell Mol Biol. 2008;39(2):235–42. doi: 10.1165/rcmb.2007-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ostberg JR, Ertel BR, Lanphere JA. An important role for granulocytes in the thermal regulation of colon tumor growth. Immunol Invest. 2005;34(3):259–72. doi: 10.1081/imm-200064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rice P, et al. Febrile-range hyperthermia augments neutrophil accumulation and enhances lung injury in experimental gram-negative bacterial pneumonia. J Immunol. 2005;174(6):3676–85. doi: 10.4049/jimmunol.174.6.3676. [DOI] [PubMed] [Google Scholar]
- 105.Chen Q, Wang WC, Evans SS. Tumor microvasculature as a barrier to antitumor immunity. Cancer Immunol Immunother. 2003;52(11):670–9. doi: 10.1007/s00262-003-0425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takeda T, et al. Hyperthermic immuno-cellular therapy-basic and clinical study. Gan To Kagaku Ryoho. 2008;35(12):2244–6. [PubMed] [Google Scholar]
- 107.Bai XF, et al. On the site and mode of antigen presentation for the initiation of clonal expansion of CD8 T cells specific for a natural tumor antigen. Cancer Res. 2001;61 (18):6860–7. [PubMed] [Google Scholar]
- 108.van Mierlo GJ, et al. Activation of dendritic cells that cross-present tumor-derived antigen licenses CD8+ CTL to cause tumor eradication. J Immunol. 2004;173(11):6753–9. doi: 10.4049/jimmunol.173.11.6753. [DOI] [PubMed] [Google Scholar]
- 109.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115(6):1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maine GN, Mule JJ. Making room for T cells. J Clin Invest. 2002;110(2):157–9. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tran KQ, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31(8):742–51. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]