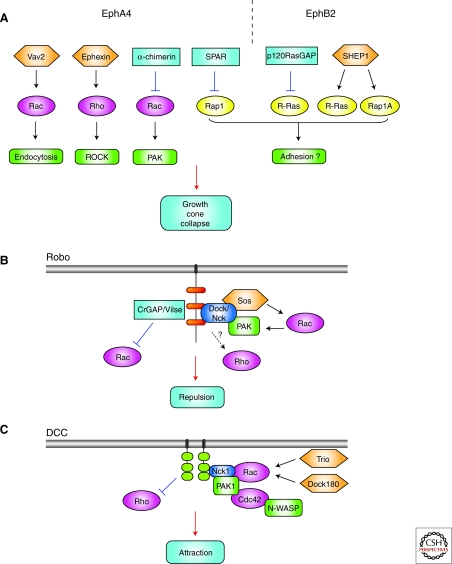
Figure 5.
Rho and Ras GTPases in Ephrin/Slit/Netrin signaling. (A) Schematic of the GEFs and GAPs involved in EphA4 (left) or EphB2 (right) signaling. Inhibition of Rac/PAK cascade (by the Rac GAP α–chimerin) may function together with ephexin-mediated Rho activation in controlling the cytoskeletal rearrangements leading to growth-cone collapse. However, Rac activation (by Vav2) can also contribute to collapse by stimulating endocytosis of the Ephrin/Eph complex. A tight regulation of R-Ras and Rap1 through GAPs (SPAR, p120RasGAP) and GEFs (like SHEP1) seems to be needed downstream of Eph signaling, possibly for the control of matrix adhesion. (B) Rac activity appears to be tightly regulated downstream of Slit through the GEF Sos and the GAP CrGAP/Vilse. Slit stimulation recruits the adaptor protein Dreadlocks (Drosophila Dock/vertebrate Nck) and subsequently PAK to Robo conserved cytoplasmic (CC) sequences (orange). The Robo/Dock complex interacts with Sos, mediating Slit-dependent Rac activation. The role of Rho in Slit/Robo signaling remains unclear and may depend on the neuronal context. (C) DCC homodimers promote growth-cone attraction through Rac, Cdc42, and PAK activation. On Netrin-1 binding, the adaptor Nck (which constitutively interacts with DCC), active Rac, Cdc42, Pak1, and N-WASP are recruited into a complex with the intracellular domain of DCC, triggering reorganization of the growth-cone actin cytoskeleton. The GEFs DOCK180 and Trio appear to be involved in netrin-1-dependent Rac activation. DCC may also down-regulate Rho and ROCK; however, the signaling mechanisms leading to the modulation of Rho activity downstream of netrin are still unclear, and they are likely to include cross talk with other GTPases. Only the intracellular domains of Robo and the DCC homodimer are shown in (B) and (C), respectively.