Abstract
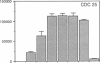
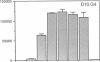
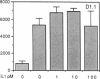
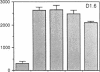
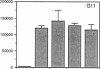
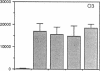
The activation of T lymphocytes requires their stimulation via clonotypic antigen receptors as well as nonantigen-specific costimulators, the best defined of which is the cytokine interleukin 1 (IL-1). Recent studies have shown that murine CD4+ helper T lymphocytes consist of two nonoverlapping subsets that selectively utilize interleukin 2 (IL-2) or interleukin 4 as their autocrine growth factors and are called Th1 and Th2 cells, respectively. We now show that IL-1 functions as a costimulator for the proliferation of Th2 but not of Th1 clones and only Th2 cells express high-affinity receptors for IL-1. Secretion of autocrine growth-promoting lymphokines by Th1 and Th2 cells occurs after stimulation via the antigen receptor-CD3 complex and is neither dependent on nor affected by IL-1. These findings suggest that the activation of T lymphocytes can be divided into two stages, lymphokine secretion and proliferation, and only proliferation requires costimulators such as IL-1. Moreover, the prevailing view that IL-1 functions as a costimulator by inducing secretion of IL-2 or expression of IL-2 receptors may not be generally applicable, because IL-2-producing Th1 clones do not express receptors for IL-1 and are insensitive to this cytokine.
Full text
PDF
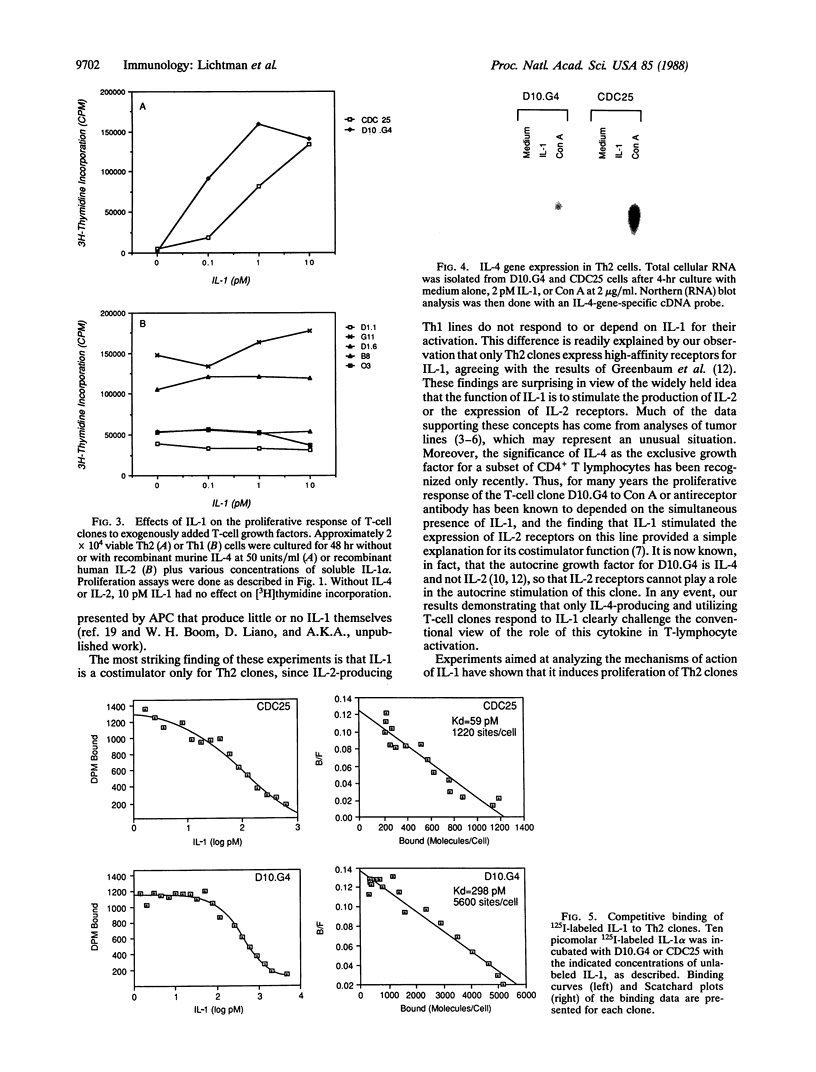
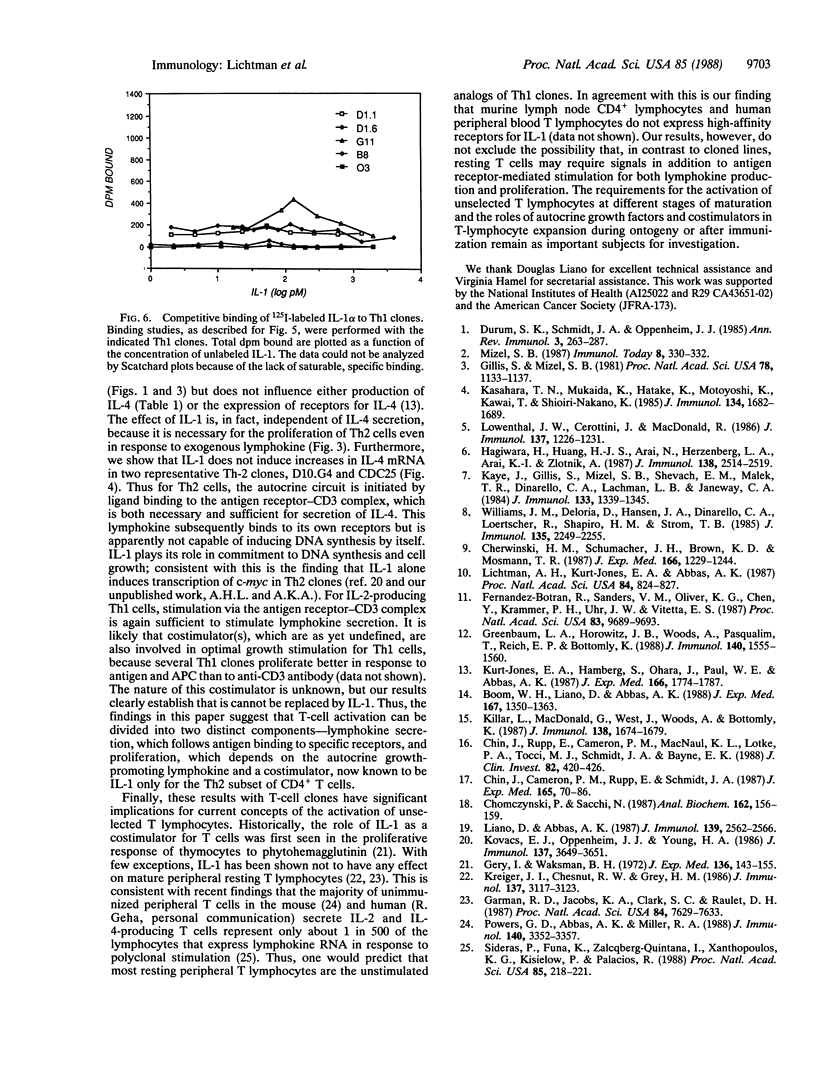
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom W. H., Liano D., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. II. Effects of interleukin 4- and interleukin 2-producing T cell clones on resting B lymphocytes. J Exp Med. 1988 Apr 1;167(4):1350–1363. doi: 10.1084/jem.167.4.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J., Cameron P. M., Rupp E., Schmidt J. A. Identification of a high-affinity receptor for native human interleukin 1 beta and interleukin 1 alpha on normal human lung fibroblasts. J Exp Med. 1987 Jan 1;165(1):70–86. doi: 10.1084/jem.165.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J., Rupp E., Cameron P. M., MacNaul K. L., Lotke P. A., Tocci M. J., Schmidt J. A., Bayne E. K. Identification of a high-affinity receptor for interleukin 1 alpha and interleukin 1 beta on cultured human rheumatoid synovial cells. J Clin Invest. 1988 Aug;82(2):420–426. doi: 10.1172/JCI113614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Oliver K. G., Chen Y. W., Krammer P. H., Uhr J. W., Vitetta E. S. Interleukin 4 mediates autocrine growth of helper T cells after antigenic stimulation. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9689–9693. doi: 10.1073/pnas.83.24.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery I., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. II. The cellular source of potentiating mediator(s). J Exp Med. 1972 Jul 1;136(1):143–155. doi: 10.1084/jem.136.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Mizel S. B. T-Cell lymphoma model for the analysis of interleukin 1-mediated T-cell activation. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1133–1137. doi: 10.1073/pnas.78.2.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum L. A., Horowitz J. B., Woods A., Pasqualini T., Reich E. P., Bottomly K. Autocrine growth of CD4+ T cells. Differential effects of IL-1 on helper and inflammatory T cells. J Immunol. 1988 Mar 1;140(5):1555–1560. [PubMed] [Google Scholar]
- Hagiwara H., Huang H. J., Arai N., Herzenberg L. A., Arai K., Zlotnik A. Interleukin 1 modulates messenger RNA levels of lymphokines and of other molecules associated with T cell activation in the T cell lymphoma LBRM33-1A5. J Immunol. 1987 Apr 15;138(8):2514–2519. [PubMed] [Google Scholar]
- Kasahara T., Mukaida N., Hatake K., Motoyoshi K., Kawai T., Shiori-Nakano K. Interleukin 1 (IL 1)-dependent lymphokine production by human leukemic T cell line HSB.2 subclones. J Immunol. 1985 Mar;134(3):1682–1689. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Killar L., MacDonald G., West J., Woods A., Bottomly K. Cloned, Ia-restricted T cells that do not produce interleukin 4(IL 4)/B cell stimulatory factor 1(BSF-1) fail to help antigen-specific B cells. J Immunol. 1987 Mar 15;138(6):1674–1679. [PubMed] [Google Scholar]
- Kovacs E. J., Oppenheim J. J., Young H. A. Induction of c-fos and c-myc expression in T lymphocytes after treatment with recombinant interleukin 1-alpha. J Immunol. 1986 Dec 1;137(11):3649–3651. [PubMed] [Google Scholar]
- Krieger J. I., Chesnut R. W., Grey H. M. Capacity of B cells to function as stimulators of a primary mixed leukocyte reaction. J Immunol. 1986 Nov 15;137(10):3117–3123. [PubMed] [Google Scholar]
- Kurt-Jones E. A., Hamberg S., Ohara J., Paul W. E., Abbas A. K. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987 Dec 1;166(6):1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liano D., Abbas A. K. Antigen presentation by hapten-specific B lymphocytes. V. Requirements for activation of antigen-presenting B cells. J Immunol. 1987 Oct 15;139(8):2562–2566. [PubMed] [Google Scholar]
- Lichtman A. H., Kurt-Jones E. A., Abbas A. K. B-cell stimulatory factor 1 and not interleukin 2 is the autocrine growth factor for some helper T lymphocytes. Proc Natl Acad Sci U S A. 1987 Feb;84(3):824–827. doi: 10.1073/pnas.84.3.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal J. W., Cerottini J. C., MacDonald H. R. Interleukin 1-dependent induction of both interleukin 2 secretion and interleukin 2 receptor expression by thymoma cells. J Immunol. 1986 Aug 15;137(4):1226–1231. [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Sideras P., Funa K., Zalcberg-Quintana I., Xanthopoulos K. G., Kisielow P., Palacios R. Analysis by in situ hybridization of cells expressing mRNA for interleukin 4 in the developing thymus and in peripheral lymphocytes from mice. Proc Natl Acad Sci U S A. 1988 Jan;85(1):218–221. doi: 10.1073/pnas.85.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]