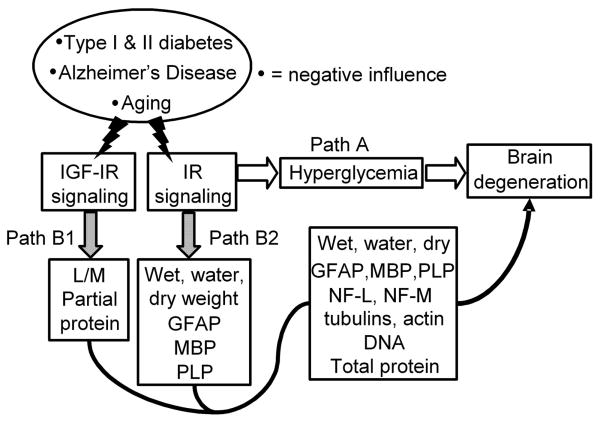
Fig. 1.
Two hypotheses for the pathogenesis of adult brain atrophy and degeneration due to concomitantly reduced signaling through the insulin as well as IGF systems. Insulin and IGF signaling are reduced with aging and further as a consequence of type 1 diabetes, type 2 diabetes or Alzheimer's disease.
“Classical” hypothesis: Reduced IR signaling via Path A glucoregulatory activity leads to hyperglycemia, increased polyol levels, accumulation of advanced glycation end (AGE) products, abnormal protein glycosylation, hyperlipemia, dehydration and other metabolic consequences that are widely believed to be pathogenic for brain atrophy and degeneration.
Alternative hypothesis: The IR signals simultaneously via Path A glucoregulatory and Path B growth factor activities. It is proposed that a concomitant decline in insulin system and IGF system signaling results in a catabolic state in brain by predominantly, albeit not necessarily exclusively, the loss of Path B growth factor activities relatively independent of Path A glucoregulation. IR signaling via Path B1 prevents the loss of brain wet, water and dry weights as well as glial fibrillary acidic protein (GFAP), myelin basic protein (MBP), and proteolipid protein (PLP) levels. IGF-I receptor signaling prevents the loss of learning and memory and partially the loss of brain protein content via Path B2 (Lupien et al., 2003). The combination of insulin and IGF may regulate brain wet, water, dry weights, DNA, total protein, GFAP, PLP, NF-L, NF-M, β-tubulin class III, α-tubulin and β-tubulin contents. The existence of Path B can be tested by the discriminating prediction that a tiny local i.c.v. dose of insulin or its combination with IGF can prevent brain atrophy and degeneration independently of unabated Path A hyperglycemia in diabetic rats.