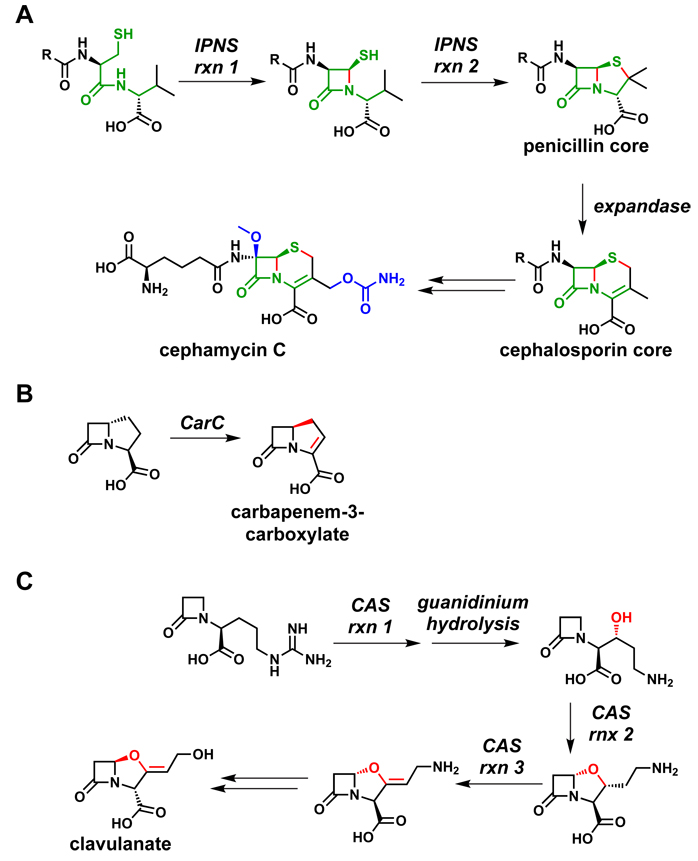
Figure 14.
Oxidative tailoring reactions from β-lactam pathways. (A) IPNS230 catalyzes two successive two-electron oxidations to form the 4,5-ring system of the penicillins. Following an epimerization reaction in the acyl chain, DAOCS231 (expandase) catalyzes the conversion of the 5-membered ring to a 6-membered ring to form the 4,6-ring system of the cephalosporins. The portion of the aminoadipoyl-Cys-Val tripeptide that becomes the β-lactam core is highlighted in green, the bonds formed by oxidation are colored red, and post-assembly modifications are shown in blue. (B) CarC catalyzes both the epimerization and the desaturation of the carbapenem core; both modifications are shown in red.232 (C) CAS catalyzes three different oxidative transformations during clavulanate biosynthesis, each of which is shown in red.233