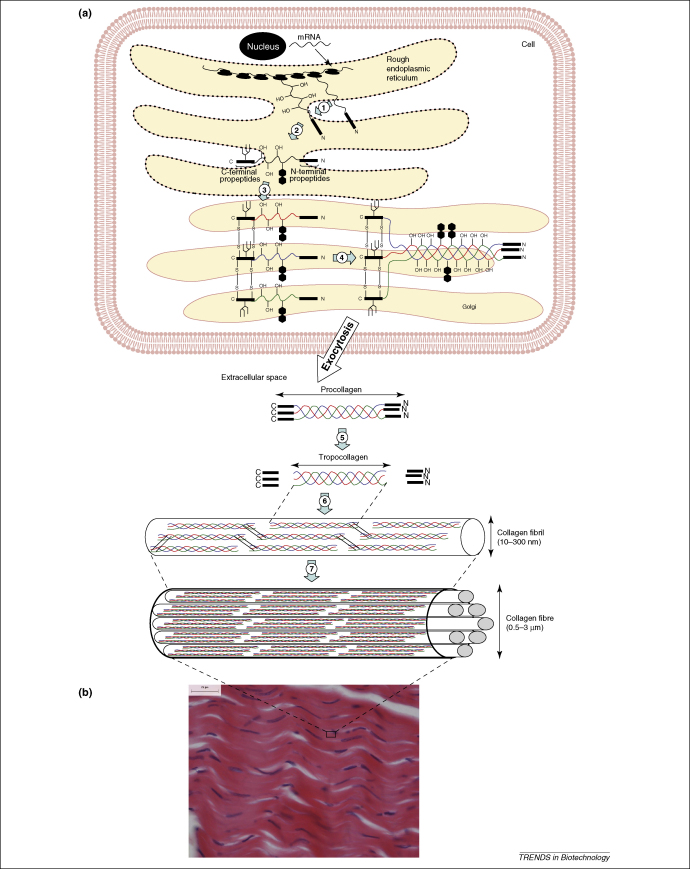
Figure 1.
Collagen biosynthesis. (a) This panel shows the major events in collagen fibre assembly. Modifications of collagen include hydroxylation of prolyl and lysyl residues (1), addition of N-linked oligosaccharides (1) and glycosylation of hydroxylysyl residues in the endoplasmic reticulum (2), before chain alignment and disulphide bond formation (3), which results in the formation of the procollagen triple helix in the Golgi (4). After export from the cell, the N- and C-terminal propeptides are cleaved (5) and the resulting tropocollagen undergoes extensive crosslinking and self-assembly into collagen fibrils of diameters between 10 and 300 nm (6). These fibres in turn further assemble into larger fibres (0.5–3 μm diameter) (7). (b) Histological section of porcine patellar tendon showing a large number of intertwined collagen fibres with ligament fibroblasts, which are stained with haemotoxylin (stains nuclei in blue-purple) and eosin (stains collagen fibres in pink). The scale bar represents 25 μm.