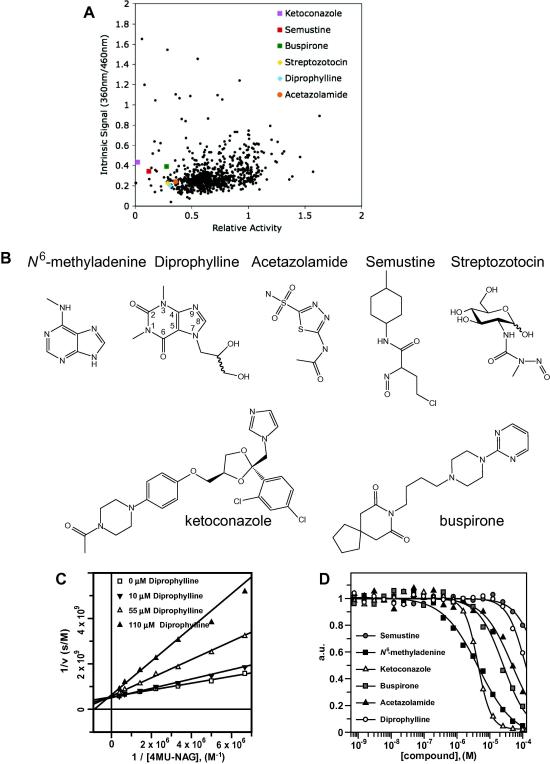
Fig. 1.
(A) Prestwick library screen against CpOGA. The scatter-plot shows on the horizontal axis the remaining activity of CpOGA in presence of the compounds (measured by the liberation of 4MU) and on the vertical axis the intrinsic absorbance/fluorescence of the compounds at the excitation and emission wavelength (360 nm/460 nm). Points in the lower left corner indicate compounds that do not absorb at the excitation wavelength, yet do exhibit reduced fluorescence and thus reduce CpOGA activity. Six compounds are labelled in coloured dots, according to the legend in the graph. (B) Chemical structures of compounds selected with the aid of the Prestwick screening data: (a) N6-methyladenine, (b) diprophylline, (c) acetazolamide, (d) semustine, (e) streptozotocin (STZ), (f) ketoconazole and (g) buspirone. (C) Diprophylline is a competitive inhibitor of CpOGA. Steady-state kinetic data (triplicates) using 0.2 nM CpOGA, 0–25 μM substrate (4MU-GlcNAc), 0–110 μ inhibitor and 7 min reaction time were fitted using the standard equation for competitive inhibition in the GraFit program [52]. (D) Dose–response curves of the selected compounds against hOGA. Data obtained with the 4MU-GlcNAc assay (2 nM hOGA, 80 μM 4MU-GlcNAc, 0.7 nM–100 μM inhibitor, 60 min reaction time) from buspirone, diprophylline and acetazolamide, semustine, N6-methyladenine and ketoconazole were fitted using the standard IC50 equation in the GraFit program [52].