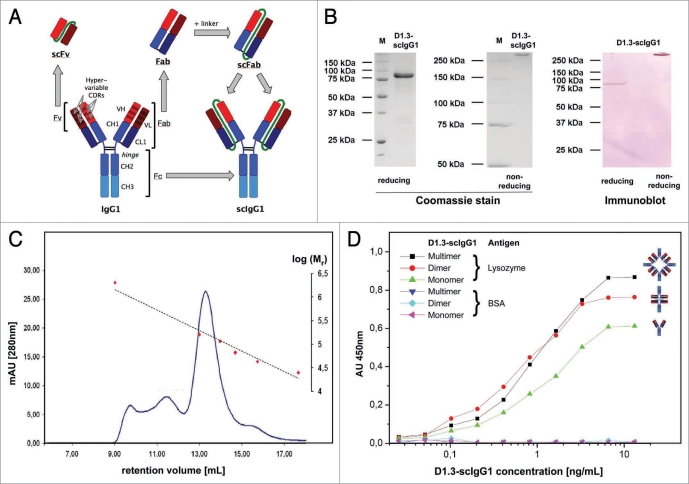
Figure 1.
(A) Schematic illustration of igG and its single chain derivatives. Variable immunoglobulin regions (VL, VH) are shown in red, constant regions (CH1, CH2, CH3) including the hinge region are shown in blue. interchain disulphide bonds are coloured black and non-sequences (linkers) are colored green. (B) SDS-pAGe and immunoblot of D1.3-scigG. After protein A affinity purification, protein samples were prepared under reducing and non-reducing conditions and separated by SDS-pAGe. Gels were stained using Coomassie Blue or blotted onto a pVDf membrane which was stained with a goat-anti-human (fc-specific) alkaline phosphate antibody conjugate (NBt-BCip substrate). (C) Size exclusion chromatography of protein A purified D1.3-scigG1. peak positions found for the molecular weight calibration standards chymotrypsinogen A (25 kDa), ovalbumin (43 kDa), albumin (67 kDa), Crp (115 kDa), igG (160 kDa) and Blue Dextran 2000 (2 MDa) are indicated in red. the dashed line indicates the deduced calibration curve. (D) Antigen eLiSA of D1.3-scigG1 SeC fractions. Serial concentrations of the D1.3-scigG1 were analysed with BSA as negative control antigen. proposed oligomerization forms suggested by the observed molecular mass are schematically illustrated.