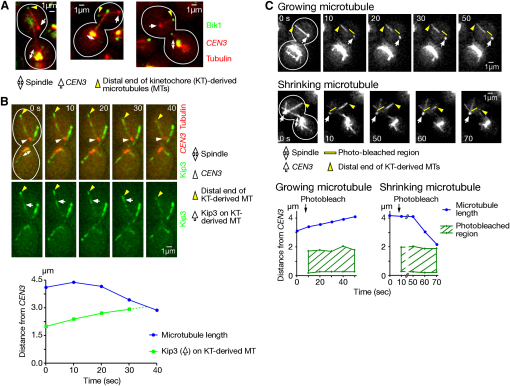
Figure 2.
Kinetochore-Derived Microtubules Have Their Plus Ends Distal to Kinetochores
(A) Bik1 signals were found at the distal ends of KT-derived MTs, but not at CEN3. Pgal-CEN3-tetOs TetR-3×CFP CFP-TUB1 BIK1-3×YFP Pmet3-CDC20 cells (T3673) were treated as in Figure 1C. YFP (Bik1; green) and CFP (CEN3, tubulin; red) images were acquired.
(B) Kip3 moved along KT-derived MTs, heading toward the end distal to CEN3. Pgal-CEN3-tetOs TetR-3×CFP CFP-TUB1 KIP3-3×GFP Pmet3-CDC20 cells (T3981) were treated as in Figure 1C. GFP (Kip3; green) and CFP (CEN3, tubulin; red) images were acquired every 10 s (time 0, arbitrary). Representative time-lapse images (top) and graphs (bottom) showing the length of the relevant KT-derived MT as well as the position of a Kip3 signal (indicated by arrows in top panels) on it.
(C) Polymerization and depolymerization of KT-derived MTs occurred at the ends distal to CEN3. Pgal-CEN3-tetOs TetR-GFP YFP-TUB1 Pmet3-CDC20 cells (T3531) were treated as in Figure 1C. A small region, close to CEN3, on a KT-derived MT was photobleached between 0 and 10 s, while the KT-derived MT was either growing or shrinking (in five and six cells, respectively). YFP (tubulin) and GFP (CEN3) signals were acquired together every 10 s (time 0, arbitrary). Representative time-lapse images (top) and graphs (bottom) showing the length of the KT-derived MTs and the positions of the photobleached regions. Other cells showed similar results. See Figure S2 in Supplemental Information.