Abstract
High-permeability pulmonary edema causing acute respiratory distress syndrome is associated with high mortality. Using a model of intratracheal adenovirus (Ad)-mediated overexpression of human vascular endothelial growth factor (VEGF)-A165 in mouse lung to induce alveolar permeability and consequent pulmonary edema, we hypothesized that systemic administration of a second adenoviral vector expressing an anti-VEGF antibody (AdαVEGFAb) would protect the lung from pulmonary edema. Pulmonary edema was induced in mice by intratracheal administration of AdVEGFA165. To evaluate anti-VEGF antibody therapy, the mice were treated intravenously with AdαVEGFAb, an adenoviral vector encoding the light and heavy chains of an anti-human VEGF antibody with the bevacizumab (Avastin) antigen-binding site. Lung VEGF-A165 and phosphorylated VEGF receptor (VEGFR)-2 levels, histology, lung wet-to-dry weight ratios, and bronchoalveolar lavage fluid (BALF) levels of total protein were assessed. Administration of AdαVEGFAb to mice decreased AdVEGFA165-induced levels of human VEGF-A165 and phosphorylated VEGFR-2 in the lung. Histological analysis of AdαVEGFAb-treated mice demonstrated a reduction of edema fluid in the lung tissue that correlated with a reduction of lung wet-to-dry ratios and BALF total protein levels. Importantly, administration of AdαVEGFAb 48 hr after induction of pulmonary edema with AdVEGFA165 was effective in suppressing pulmonary edema. Administration of an adenoviral vector encoding an anti-VEGF antibody that is the equivalent of bevacizumab effectively suppresses VEGF-A165-induced high-permeability pulmonary edema, suggesting that anti-VEGF antibody therapy may represent a novel therapy for high-permeability pulmonary edema.
Introduction
Pulmonary edema, a significant cause of morbidity and mortality in a critical care setting, is characterized by excessive extravascular fluid in the lungs (Staub, 1974; Fraser et al., 1999; Matthay et al., 2002; Matthay and Martin, 2005). The alteration of fluid balance in the lung that results in pulmonary edema can be caused by increased hydrostatic pressure or increased permeability of the pulmonary capillary bed (Guyton and Lindsey, 1959; Gorin and Stewart, 1979; Montaner et al., 1986; Matthay et al., 2002). High-permeability pulmonary edema results from an increase in the vascular permeability of the lung, causing an accumulation of fluid and protein in the lung interstitium and air spaces (Gorin and Stewart, 1979; Montaner et al., 1986; Matthay et al., 2002). The increase in permeability can be initiated by a variety of factors, and is directly related to reversible physical modifications of the pulmonary endothelium (Fraser et al., 1999; Matthay and Martin, 2005; Leaver and Evans, 2007; Wheeler and Bernard, 2007).
Various experimental and human studies support the concept of vascular endothelial growth factor (VEGF) playing an important role in the pathogenesis of high-permeability pulmonary edema (Becker et al., 2000; Kaner et al., 2000; Thickett et al., 2001; Karmpaliotis et al., 2002; Carpenter et al., 2003, 2005; Choi et al., 2003; Gurkan et al., 2003; Lee et al., 2004; Bhandari et al., 2006, 2008; Godzich et al., 2006; Kunig et al., 2006). VEGF was originally described as a “vascular permeability factor” related to its ability to induce permeability in guinea pig skin (Senger et al., 1983; Dvorak et al., 1995). It is now recognized that VEGF is a critical regulator of vascular permeability in several organs (Roberts and Palade, 1995; Dvorak, 2006; Breen, 2007). The lung is particularly vulnerable, as there are 500-fold higher levels of VEGF in normal human lung epithelial lining fluid (ELF) than in human plasma (Kaner and Crystal, 2001); if the tight junctions of the respiratory epithelium are disturbed, the high concentrations of VEGF in lung ELF can reach the alveolar capillary endothelium, interact with the endothelial cell VEGF receptors, and induce vascular permeability (Kaner and Crystal, 2004; Mura et al., 2004; Voelkel et al., 2006; Gropper and Wiener-Kronish, 2008; Kosmidou et al., 2008). Overexpression of human VEGF-A165 by intratracheal administration of an adenovirus (Ad)-based gene transfer vector (AdVEGFA165) to mice causes pulmonary edema and increased vascular permeability, suggesting a significant role for VEGF in the development of pulmonary edema (Kaner et al., 2000).
Bevacizumab (Avastin), a humanized IgG1 monoclonal antibody specific for human VEGF-A, is effective at inhibiting VEGF-dependent processes (Ferrara et al., 2004, 2005). Bevacizumab binds to all VEGF-A isoforms, and prevents VEGF-A from activating the two major VEGF receptors, VEGF receptor (VEGFR)-1 (Flt-1) and VEGFR-2 (KDR). In this study, we hypothesized that genetic delivery of an anti-VEGF antibody should be effective in suppressing VEGF-A165-induced high-permeability pulmonary edema. To assess this, we used an adenoviral gene transfer vector (AdαVEGFAb) expressing the heavy and light chains of a monoclonal antibody with a VEGF-A165 antigen recognition site identical to bevacizumab to suppress human VEGF-A165-induced pulmonary edema. The data demonstrate that a single intravenous administration of AdαVEGFAb is effective in reducing AdVEGFA165-induced pulmonary edema, including high-permeability pulmonary edema initiated 48 hr before AdαVEGFAb administration.
Materials and Methods
Adenoviral vectors
All adenoviral vectors were replication-deficient recombinant adenovirus type 5-based vectors with E1 and E3 deletions and under the control of the cytomegalovirus promoter/enhancer (Hackett and Crystal, 2008). AdαVEGFAb expresses a full-length antibody that has specificity for human VEGF-A (Watanabe et al., 2008). The expression cassette in AdαVEGFAb encodes the anti-human VEGF light chain and heavy chain sequence separated by a poliovirus internal ribosome entry site (IRES) to facilitate expression of both protein subunits from a single promoter (Watanabe et al., 2008). From 5′ to 3′, the expression cassette in the AdαVEGFAb vector contains the cytomegalovirus promoter/enhancer, the anti-human VEGF light chain-coding sequence, the poliovirus IRES, the anti-human VEGF heavy chain-coding sequence, and the simian virus 40 polyadenylation signal. Synthetic antibody heavy and light chain variable domains selected for the study were derived from the protein sequence for antibody A.4.6.1, the murine antibody that was humanized to generate bevacizumab (Kim et al., 1992). The coding sequences for the VEGF-binding site were identical to that of bevacizumab (Baca and Wells, 1997). The variable domains were incorporated into full-length heavy and light chains by adding murine IgG1 constant domain and the murine κ constant domain onto the variable regions by overlap polymerase chain reaction (PCR). AdVEGFA165 expresses the human VEGF-A165 cDNA (Muhlhauser et al., 1995; Kaner et al., 2000). Concerning the negative control vectors, AdαPAAb encodes an unrelated antibody against anthrax protective antigen (De et al., 2008), AdLacZ encodes β-galactosidase (Hersh et al., 1995), and AdNull encodes no transgene (Hersh et al., 1995). Propagation, purification, and titration of the adenoviral vectors was as previously described (Rosenfeld et al., 1991, 1992; Mittereder et al., 1996).
Anti-VEGF antibody levels after AdαVEGFAb administration
Male C57BL/6 mice, 8 to 10 weeks of age, were obtained from The Jackson Laboratory (Bar Harbor, ME) or Taconic (Germantown, NY) and were housed under pathogen-free conditions. To determine which administration route of the AdαVEGFAb vector would deliver the highest levels of anti-VEGF antibodies to the lung, AdαVEGFAb (1011 particle units, PU) in 100 μl of phosphate-buffered saline, pH 7.4 (PBS) was administered by the intravenous, intratracheal, or intrapleural route to C57BL/6 mice. After 1 to 140 days, bronchoalveolar lavage fluid (BALF) and serum were collected. BALF was collected by cannulating the trachea with a 24-gauge angiocatheter and flushing and aspirating three times with 500 μ1 of PBS, and then centrifuged at 3500 rpm for 5 min. Blood was collected via the tail vein, allowed to clot for 60 min, and centrifuged at 13,000 rpm for 10 min. Anti-human VEGF antibody levels in mouse BALF and serum were assessed by a human VEGF-specific enzyme-linked immunosorbent assay (ELISA) using flat-bottomed 96-well EIA/RIA plates (Corning Life Sciences, Lowell, MA) coated with 0.1 μg of human VEGF-A165 per well in a total volume of 100 μl of 0.05 M carbonate buffer containing 0.01% thimerosal overnight at 4°C. The plates were washed three times with PBS and blocked with 5% dry milk in PBS for 30 min. The plates were washed three times with PBS containing 0.05% Tween 20 (PBS–Tween). Serial serum dilutions in PBS containing 1% dry milk were added to each well and incubated for 60 min. The plates were washed three times with PBS–Tween and 100 μl/well of 1:10,000 diluted horseradish peroxidase-conjugated goat anti-mouse IgG1 (Santa Cruz Biotechnology, Santa Cruz, CA) in PBS containing 1% dry milk was added and incubated for 60 min. The plates were washed four times with PBS–Tween and once with PBS. Peroxidase substrate (100 μl/well; Bio-Rad, Hercules, CA) was added; after 10 min, the reaction was stopped by addition of 2% oxalic acid (100 μl/well). Absorbance at 415 nm was measured. Antibody titers were calculated with a log (optical density)–log (dilution) interpolation model and a cutoff value equal to 2-fold the absorbance of background (Plikaytis et al., 1991) and normalized to total protein using a bicinchoninic acid protein assay kit (Thermo Scientific, Rockford, IL).
Organ distribution of anti-VEGF antibody mRNA expression levels after intravenous AdαVEGFAb administration
Quantitative TaqMan real-time PCR analysis was used to evaluate organ distribution of anti-VEGF antibody mRNA expression levels after intravenous AdαVEGFAb (1011 PU) administration. Primers and probe for the anti-VEGF antibody gene were 5′-GGTCTTAAGTGGATGGGATGGA-3′, 5′-TGTGAACCTGCGCTTGAAATC-3′, and FAM-TAATACTTATACTGGAGAACCTACCTACGCTG-TAMRA, respectively. Five days after vector administration, liver, lung, heart, spleen, and kidney were collected and total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA) followed by DNA digestion with deoxyribonuclease I, amplification grade (Invitrogen). First-strand cDNA was synthesized from 1 μg of total RNA in a 50-μl reaction volume, using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) with random hexamers as primers for RT-PCR. Quantitative TaqMan real-time PCR relative to an 18S ribosomal RNA (rRNA) primer and probe set as the internal control (Applied Biosystems) was performed to assess the expression levels of anti-VEGF antibody mRNA in the sample, using the ΔΔCt method.
Pulmonary edema therapy model
AdVEGFA165, 1011 PU in 100 μl of PBS, was administered intratracheally to mice to induce edema as previously described (Kaner et al., 2000). In this prior study, evaluation of the time course of edema formation demonstrated a peak of the lung wet-to-dry ratio between days 3 and 5, but there was a significant increase in the ratio relative to animals receiving a control vector throughout a 10-day time course. As a negative control, AdLacZ or AdNull, 1011 PU in 100 μl of PBS, was administered intratracheally to mice. AdαVEGFAb, 1011 PU in 100 μl of PBS, was administered intravenously either at the same time as AdVEGFA165, or at various times after AdVEGFA165 administration. As a negative control, AdNull or AdαPAAb, 1011 PU in 100 μl of PBS, was administered intravenously at the same time points. PBS was an additional negative control.
Lung wet-to-dry weight ratio
Five days after AdVEGFA165 administration, lungs were excised en bloc and dissected away from the heart and thymus. The lungs were immediately weighed and then placed in a desiccating oven at 65°C for 48 hr, at which point dry weight was achieved. The ratio of lung wet-to-dry weight was used to quantify lung water content (Staub, 1974; Kaner et al.,2000).
Assessment of lung VEGF-A165 levels and VEGFR-2 phosphorylation
BALF was collected and centrifuged as described previously. Human VEGF-A165 levels in BALF were measured by an ELISA (R&D Systems, Minneapolis, MN) with a human VEGF-specific primary antibody that has no cross-reactivity with mouse VEGF. Phosphorylation of VEGFR-2 was assessed by Western analysis. Frozen whole lung tissues were homogenized in 300 μl of protein extraction reagent (Thermo Scientific) with protease and phosphatase inhibitors. The homogenates were centrifuged at 13,000 rpm for 20 min at 4°C, and the supernatants were collected. Pooled protein (100 μg from n = 3 mice per group) was evaluated for total VEGFR-2 levels with rabbit anti-VEGFR-2 (Cell Signaling Technology, Danvers, MA) as the primary antibody and a horseradish peroxidase-linked donkey anti-rabbit IgG antibody (GE Healthcare Life Sciences, Piscataway, NJ) as the secondary antibody. The same samples were analyzed for phosphorylation of VEGFR-2, using HRP-conjugated mouse anti-phosphotyrosine clone 4G10 (Millipore, Billerica, MA) as the primary antibody. Detection was with ECL reagent (GE Healthcare Life Sciences). The intensities of the bands were analyzed with MetaMorph image analysis software (Universal Imaging, Downingtown, PA). Immunoprecipitation with the anti-VEGFR-2 antibody was done to enrich for VEGFR-2 before phosphotyrosine immunoblotting; however, this was not an improvement over direct Western analysis, likely because of the insensitivity of the anti-VEGFR-2 antibody.
Lung histopathology
To assess lung histopathology, mice were exsanguinated, and 2 ml of PBS was injected via the jugular vein to perfuse the lungs. Lungs were inflated to total lung capacity with air injection via an angiocatheter placed in the trachea and tied with sutures. The air-inflated lungs were removed en bloc, placed in an uncovered container with 300 ml of PBS, and submerged by covering with saturated gauze. The PBS was heated to 60°C, 4 min in a microwave oven (Turner et al., 1990). The lungs were then fixed in 4% paraformaldehyde followed by paraffin embedding.
Measurement of BALF total protein levels
BALF was collected as described previously at various time points and under conditions as detailed in text. Photos were taken of the lavage fluid before centrifugation. The BALF was then centrifuged to remove blood proteins and total protein levels were measured by the bicinchoninic method (Thermo Scientific) as specified by the manufacturer.
Statistical analysis
All data are shown as means ± standard error. Statistical comparison was made by a two-tailed Student t test, and a value of p < 0.05 was accepted as indicating significance.
Results
Comparison of serum and BALF anti-VEGF antibody levels after administration of AdαVEGFAb by different routes
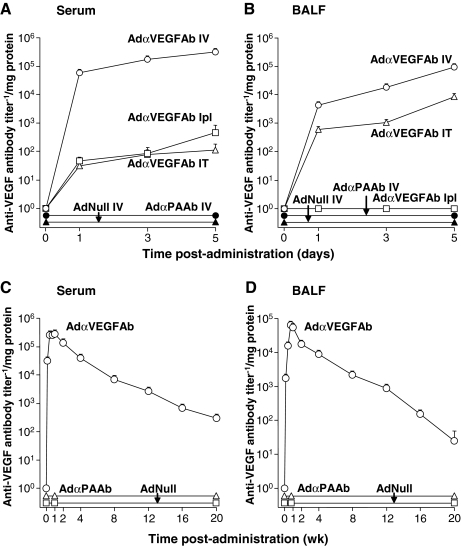
To determine the administration route of AdαVEGFAb required to achieve the highest lung anti-VEGF levels, BALF and serum anti-VEGF antibody levels were assessed after intravenous, intratracheal, or intrapleural administration of the same dose of the vector. Intravenous AdαVEGFAb administration resulted in the highest antibody expression levels in both serum (Fig. 1A) and BALF (Fig. 1B) compared with intratracheal or intrapleural administration (p < 0.0001). No anti-VEGF antibody was detected in the BALF and serum from mice that received intravenous administration of the control AdαPAAb or AdNull vector. Assessment of the expression profile of anti-VEGF antibody levels in serum and BALF over 20 weeks after intravenous AdαVEGFAb administration showed that serum and BALF anti-VEGF antibody levels peaked on days 7 and 5, respectively, and then gradually decreased over time (Fig. 1C and D).
FIG. 1.
Comparison of serum and bronchoalveolar lavage fluid (BALF) anti-vascular endothelial growth factor (VEGF) antibody levels after administration of the AdαVEGFAb vector by different routes. (A and B) Time course of serum and BALF anti-VEGF antibody levels after vector administration by different routes. AdαVEGFAb (1011 particle units [PU]) was administered to C57BL/6 mice by the intravenous (IV), intratracheal (IT), or intrapleural (Ipl) route. AdαPAAb and AdNull were administered intravenously as controls. (A) Serum anti-VEGF antibody levels. (B) BALF anti-VEGF antibody levels. (C and D) Anti-VEGF antibody levels in serum and BALF over 20 weeks after intravenous administration of AdαVEGFAb. (C) Serum anti-VEGF antibody levels. (D) BALF anti-VEGF antibody levels. AdαPAAb and AdNull were administered intravenously as controls. For all panels, for direct comparison, data are presented relative to total protein levels in serum or BALF, respectively. Data for each panel were obtained from n = 5 animals per group.
Organ distribution of anti-VEGF antibody mRNA expression levels after intravenous AdαVEGFAb administration
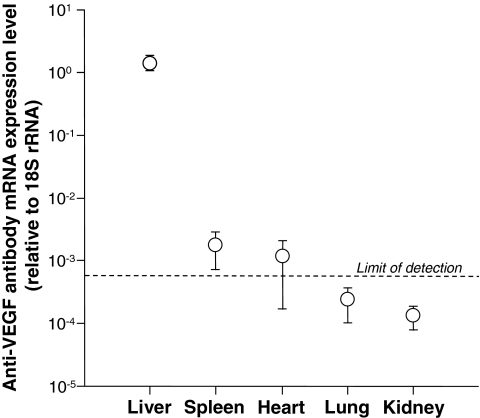
To evaluate the organ distribution of anti-VEGF antibody mRNA expression levels after intravenous AdαVEGFAb administration, anti-VEGF antibody mRNA expression levels relative to endogenous 18S rRNA in various tissues were assessed by quantitative TaqMan real-time PCR. The highest amount of anti-VEGF antibody mRNA expression level was found in the liver, followed by the spleen (Fig. 2).
FIG. 2.
Organ distribution of anti-VEGF antibody mRNA expression levels after intravenous administration of AdαVEGFAb. AdαVEGFAb (1011 PU) was administered intravenously. On day 5, various organs were collected and anti-VEGF antibody mRNA expression levels relative to endogenous 18S rRNA were assessed by quantitative TaqMan real-time PCR (limit of detection, 0.000567). Data were obtained from n = 2 animals per group.
Effects of intratracheal AdVEGFA165 administration on lung wet-to-dry weight ratio
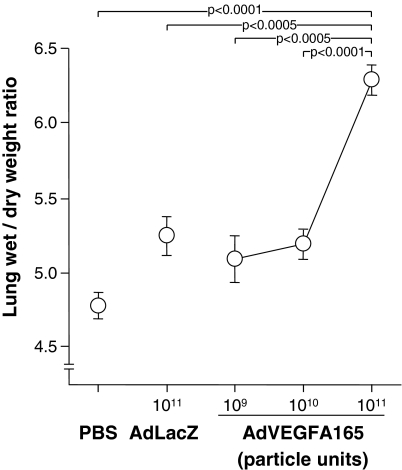
The lung wet-to-dry weight ratio, a measure of extravascular lung water, was quantified as a function of AdVEGFA165 dose after intratracheal administration. In previous studies, we have demonstrated that after intratracheal adenoviral vector administration to the epithelial surface of the respiratory tract, the respiratory epithelium is the only category of cells that express the transgene (Mastrangeli et al.,1993). The lung wet-to-dry ratio showed a dose-dependent increase, with the highest dose (1011 PU) resulting in a significant increase over that observed with AdLacZ at the same dose or with PBS (Fig. 3; p < 0.0005 compared with all other groups). Although AdLacZ administration resulted in a slightly elevated lung wet-to-dry ratio relative to PBS (9.8%), the lung wet-to-dry ratio induced by AdVEGFA165 is statistically significant relative to the same dose of AdLacZ. On the basis of these data, intratracheal AdVEGFA165, at a dose of 1011 PU, was used for all subsequent experiments to initiate high-permeability pulmonary edema.
FIG. 3.
Lung wet-to-dry weight ratio after intratracheal administration of AdVEGFA165. Increasing doses (109, 1010, or 1011 PU) of AdVEGFA165 or AdLacZ, or PBS, were administered intratracheally on day 0. On day 5, the lung wet-to-dry ratio was quantified. Data were obtained from n = 5 animals per group.
Effects of AdαVEGFAb on lung VEGF-A165 levels and VEGFR-2 phosphorylation
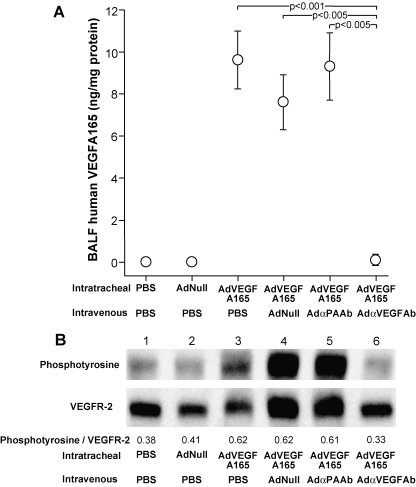
To evaluate the effect of intravenous administration of AdαVEGFAb on lung VEGF-A165 and VEGFR-2 signaling in vivo induced by intratracheal administration of AdVEGFA165, BALF levels of human VEGF-A165 and lung tissue VEGFR-2 phosphorylation levels were assessed. Treatment with AdαVEGFAb induced a significant reduction of BALF levels of human VEGF-A165 (Fig. 4A; p < 0.005, AdαVEGFAb compared with all control treated groups) and downregulation of VEGFR-2 phosphorylation (Fig. 4B) compared with AdαPAAb-, AdNull-, or PBS-treated mice.
FIG. 4.
Effects of intravenous administration of AdαVEGFAb on lung VEGFR-2 signaling induced by intratracheal administration of AdVEGFA165. AdVEGFA165 (1011 PU) was administered intratracheally and at the same time AdαVEGFAb or controls AdαPAAb, AdNull, or PBS was administered intravenously (1011 PU). On day 5, BALF and lung tissues were collected. (A) BALF human VEGF-A165 levels after intratracheal administration of AdVEGFA165 without and with parallel intravenous administration of AdαVEGFAb. Human VEGF-A165 levels are normalized to total protein. Data were obtained from n = 4 or 5 animals per group. (B) Lung tissue VEGFR-2 phosphorylation levels after intratracheal administration of AdVEGFA165 without and with parallel intravenous administration of AdαVEGFAb. Lung homogenates were assessed for total VEGFR-2 expression and phosphorylated VEGFR-2 levels by Western analysis with rabbit anti-VEGFR-2 and horseradish peroxidase (HRP)-conjugated mouse anti-phosphotyrosine antibodies. The mean intensities of the bands (phosphotyrosine/VEGFR-2) were analyzed with image analysis software. Lane 1, PBS + PBS; lane 2, AdNull + PBS; lane 3, AdVEGFA165 + PBS; lane 4, AdVEGFA165 + AdNull; lane 5, AdVEGFA165 + AdαPAAb; lane 6, AdVEGFA165 + AdαVEGFAb. Data were obtained from pooled samples from n = 3 animals per group.
Effects of AdαVEGFAb on VEGF-A165-mediated high-permeability pulmonary edema
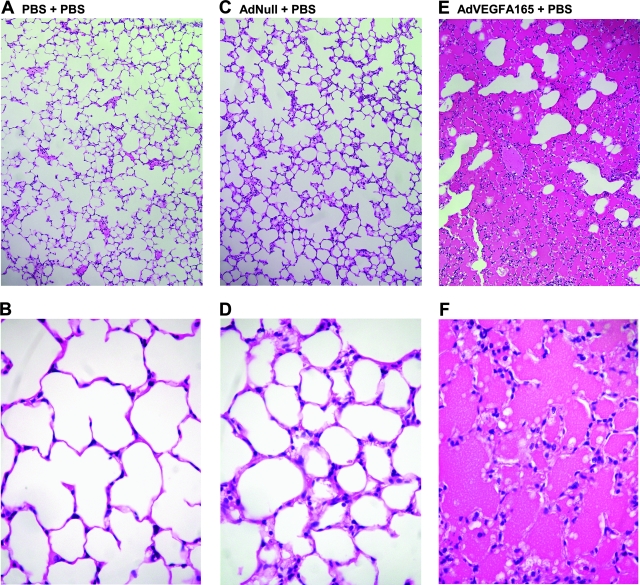
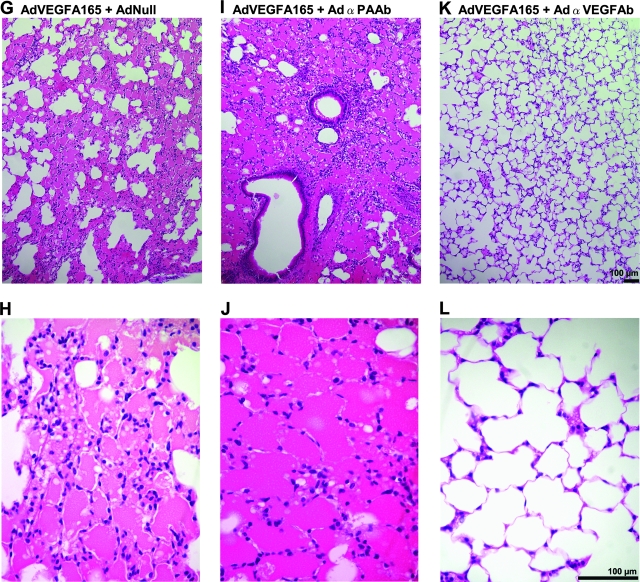
To determine the effects of AdαVEGFAb on VEGF-A165-mediated high-permeability pulmonary edema, AdVEGFA165 was administered intratracheally to mice concurrently treated by intravenous injection of AdαVEGFAb, control vectors, AdαPAAb or AdNull, or PBS. For these experiments, we did not include a direct comparison of the effect of AdαVEGFAb with the effect of purified anti-VEGF antibody; the differences in half-lives between AdαVEGFAb-expressed antibody and purified antibody, and the differences in antibody distribution after intravenous administration of AdαVEGFAb versus purified antibody, would make data interpretation and a meaningful comparison difficult. Treatment with AdαVEGFAb resulted in a marked reduction of intraalveolar edema as assessed by lung histopathology in AdαVEGFAb-treated mice as compared with the AdαPAAb-, AdNull-, or PBS-treated mice, whose alveoli were filled with bloody edema fluid (Fig. 5). The lung wet-to-dry weight ratio demonstrated a significant ratio reduction in AdαVEGFAb-treated mice relative to animals that received AdαPAAb, AdNull, or PBS (Fig. 6; p < 0.0001, AdαVEGFAb compared with all control treated groups and similar to naive animals, p > 0.5).
FIG. 5.
Lung histopathology after intratracheal administration of AdVEGFA165 and treatment with AdαVEGFAb. AdVEGFA165 (1011 PU) was administered intratracheally and at the same time AdαVEGFAb or control AdαPAAb, AdNull, or PBS was administered intravenously (1011 PU). On day 5, lungs were fixed by the microwave technique and paraffin sections were stained with hematoxylin and eosin. (A and B) PBS + PBS; (C and D) AdNull + PBS; (E and F) AdVEGFA165 + PBS; (G and H) AdVEGFA165 + AdNull; (I and J) AdVEGFA165 + AdαPAAb; and (K and L) AdVEGFA165 + AdαVEGFAb. Scale bars [shown in (K) and (L)]: 100 μm.
FIG. 6.
Lung wet-to-dry weight ratio after intratracheal administration of AdVEGFA165 and treatment with AdαVEGFAb. AdVEGFA165 (1011 PU) was administered intratracheally and at the same time AdαVEGFAb or control AdαPAAb, AdNull, or PBS was administered intravenously (1011 PU). On day 5, the lung wet-to-dry ratio was quantified. Data were normalized according to the mean level of the PBS + PBS group. Data were obtained from n = 15 animals per group.
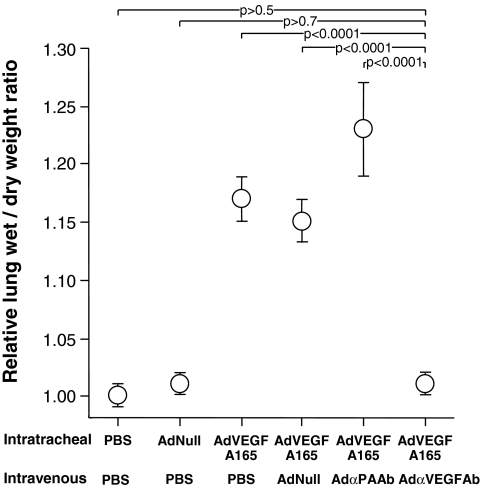
Assessment of BALF recovered from mice receiving intratracheal AdVEGFA165 and treated with AdαPAAb, AdNull, or PBS showed that BALF was bloody in all cases. In contrast, BALF from AdαVEGFAb-treated mice was similar to that of naive animals (Fig. 7A). To evaluate the effect of AdαVEGFAb on alveolar capillary protein leak induced by AdVEGFA165, BALF total protein levels were quantified. Intravenous administration of AdαVEGFAb resulted in a significant reduction of total protein levels in BALF relative to animals that received AdαPAAb, AdNull, or PBS (Fig. 7B; p < 0.005, AdαVEGFAb compared with all control groups).
FIG. 7.
BALF total protein levels after intratracheal administration of AdVEGFA165 and treatment with AdαVEGFAb. AdVEGFA165 (1011 PU) was administered intratracheally to mice and at the same time AdαVEGFAb or control AdαPAAb, AdNull, or PBS was administered intravenously (1011 PU). On day 5, BALF was collected and total protein levels were measured. (A) Photos of BALF under various conditions before centrifugation. (B) BALF total protein levels after centrifugation. Data were obtained from n = 4 or 5 animals per group.
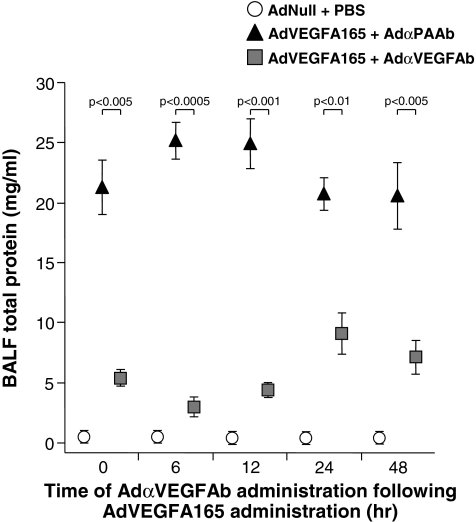
To determine the effects of delayed administration of AdαVEGFAb on VEGF-A165-mediated high-permeability pulmonary edema, AdVEGFA165 was administered intratracheally to mice and then treated by intravenous injection of AdαVEGFAb or the control vector AdαPAAb 0, 6, 12, 24, or 48 hr later; animals were killed on day 5 after AdVEGFA165 administration for analysis of BALF total protein levels. Delayed administration of AdαVEGFAb resulted in a marked reduction of BALF total protein levels relative to animals that received AdαPAAb at all time points tested (Fig. 8; p < 0.01, AdαVEGFAb compared with control groups), consistent with the concept that AdαVEGFAb was an effective treatment after the initiation of pulmonary edema.
FIG. 8.
Suppression of AdVEGFA165-induced pulmonary edema by administration of AdαVEGFAb after the pulmonary edema is induced. Shown is the BALF total protein level after intratracheal administration of AdVEGFA165 and treatment with AdαVEGFAb 0 to 48 hr afterward. AdVEGFA165 (1011 PU) was administered intratracheally to mice and, at various times ranging from 6 to 48 hr later, AdαVEGFAb or a control vector, AdαPAAb, was administered intravenously (1011 PU). On day 5, BALF was collected and centrifuged, and total protein levels were measured. Data were obtained from n = 3 animals per group.
Discussion
Pulmonary edema is a common clinical problem with significant consequences for lung mechanical functions and gas exchange (Fraser et al., 1999; Matthay and Martin, 2005; Leaver and Evans, 2007; Wheeler and Bernard, 2007). VEGF has been implicated in a variety of conditions characterized by vascular leakage, including pulmonary edema (Kaner et al., 2000; Ferrara, 2004; Mura et al., 2004; Dvorak, 2006; Voelkel et al., 2006; Breen, 2007; Kosmidou et al., 2008). In the present study, we tested the hypothesis that an anti-VEGF antibody, the murine equivalent of bevacizumab with an anti-human VEGF-binding site, would inhibit VEGF-induced high-permeability pulmonary edema. The results indicate that delivery of an anti-VEGF antibody with an adenoviral gene transfer vector (AdαVEGFAb) to mice is effective in suppressing AdVEGFA165-induced high-permeability pulmonary edema as measured by human VEGF-A165 levels in BALF, lung phosphorylated VEGFR-2 levels, histology, lung wet-to-dry weight ratios, and BALF total protein levels. Importantly, and relevant to the possible clinical application of this strategy to treat pulmonary edema, administration of AdαVEGFAb 6 to 48 hr after induction of pulmonary edema with AdVEGFA165 was successful in preventing the pulmonary edema.
VEGF and high-permeability pulmonary edema
VEGF belongs to a gene family that includes VEGF isotypes A–E and placenta growth factor, proteins that have multiple diverse roles and biological functions (Ferrara et al., 2003; Ferrara, 2004). VEGF-A is the prototype member of the family that arises from alternative splicing of the eight-exon VEGF gene to yield four isoforms of 121, 165, 189, or 206 amino acids (Tischer et al., 1991; Ferrara et al., 2003; Ferrara, 2004). VEGF-A165, the most common isoform, binds to VEGFR-1 and VEGFR-2 and induces receptor dimerization and phosphorylation of specific tyrosine residues for functional signal transduction (Matsumoto and Claesson-Welsh, 2001; Kowanetz and Ferrara, 2006; Olsson et al., 2006).
VEGF is a potent stimulus of growth of vascular endothelial cells, and this activity is relevant to recruiting a vasculature to developing tumors (Folkman, 1971; Ferrara, 2002). VEGF is also a permeability factor, capable of inducing vascular leakage (Senger et al., 1983; Dvorak et al., 1995; Roberts and Palade, 1995; Ferrara, 2004; Dvorak, 2006; Breen, 2007). In this regard, VEGF induces increased vascular permeability in multiple organs, including skin, muscle, gastrointestinal tract, CNS, and lung (Senger et al., 1983; Roberts and Palade, 1995; Bates et al., 1999; Proescholdt et al., 1999; Kaner et al., 2000; Schoch et al., 2002; Rosenstein and Krum, 2004; Breen, 2007). VEGF-dependent increases in permeability and fenestration have been observed in large vessels (Hippenstiel et al., 1998), microvascular vessels (Esser et al., 1998), and cultured endothelial cells (Bates et al., 1999). Many of the effects of VEGF on endothelial cells are related to the VEGF-induced production of endothelial cell nitric oxide (Fukumura et al., 2001).
Several animal studies have demonstrated a role for VEGF in the development of high-permeability pulmonary edema (Mura et al., 2004; Voelkel et al., 2006; Kosmidou et al., 2008). When administered intratracheally to the lung, an adenoviral gene transfer vector expressing VEGF promotes pulmonary edema and vascular leakage, and this effect is abrogated by pretreatment with an adenoviral vector expressing sFlt-1, the soluble form of VEGFR-1 (Kaner et al., 2000). In ferrets (Becker et al., 2000) and rats (Choi et al., 2003), ventilator-induced lung injury is associated with elevated lung or serum VEGF levels and vascular leak. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in rats after ischemia–reperfusion injury (Godzich et al., 2006). In a murine model of pulmonary edema induced by lipopolysaccharide inhalation, there are elevated lung levels of VEGF (Karmpaliotis et al., 2002). The VEGFR-2 levels in lung homogenate are upregulated in a murine model of pulmonary edema after intratracheal hydrochloric acid-induced lung injury, and these levels were further elevated under conditions of high tidal volume ventilation (Gurkan et al., 2003). Inhibition of VEGF activity with a soluble VEGF-Trap decoy receptor markedly reduces pulmonary vascular protein extravasation in hypoxic endothelin-B receptor-deficient rats (Carpenter et al., 2003), consistent with the concept that increased VEGF expression in the lung contributes to vascular permeability. Endothelin-driven increases in lung VEGF levels were associated with the development of pulmonary edema (Carpenter et al., 2005). Administration of recombinant human VEGF during hyperoxia-induced acute lung injury transiently increases pulmonary edema (Kunig et al., 2006). Finally, lung-targeted VEGF-A165 transgenic mice develop pulmonary edema after induction of VEGF-A165 expression (Lee et al., 2004; Bhandari et al., 2006, 2008).
In humans, elevated plasma VEGF levels are present in patients with acute respiratory distress syndrome (ARDS) as well as patients at risk for developing ARDS (Thickett et al., 2001). In this study, there was a correlation between VEGF levels and disease outcome, with higher VEGF levels in nonsurvivors.
Bevacizumab (Avastin)
Bevacizumab binds specifically to all human VEGF isoforms and bioactive proteolytic fragments, but not to mouse or rat VEGF, and prevents the binding of VEGF to its receptors (Ferrara et al., 2004, 2005). In preclinical studies, inhibiting VEGF activity with bevacizumab effectively suppresses tumor growth in immunodeficient mice with human tumor xenografts (Presta et al., 1997; Sweeney et al., 2001; Fox et al., 2002). In clinical studies, bevacizumab, in combination with standard chemotherapy regimens, significantly prolongs the survival of patients with metastatic cancers of the colon, breast, kidney, and lung (Yang et al., 2003; Hurwitz et al., 2004; Miller et al., 2005; Sandler et al., 2006). The efficacy of bevacizumab as a cancer therapy led to its approval by the U.S. Food and Drug Administration in 2004 as the first antiangiogenic agent for the treatment of cancer (Ferrara et al., 2004).
The murine anti-VEGF monoclonal antibody A.4.6.1 is the precursor to the humanized form of the antibody bevacizumab (Kim et al., 1992; Fox et al., 2002). Humanization of A.4.6.1 to reduce immunogenicity and to increase its half-life in humans resulted in an antibody molecule that is 93% human and 7% murine (Baca et al., 1997; Fox et al., 2002); both antibodies are specific for human VEGF and both are effective inhibitors of tumor growth in vivo (Gerber and Ferrara, 2005).
The activity of bevacizumab is comparable to that of other VEGF inhibitors, such as soluble VEGF receptors, that have higher binding affinity for VEGF (Kuo et al., 2001; Holash et al., 2002; Ferrara et al., 2004). These effects may be related to the relatively longer half-life of the antibody, biodistribution, or stability of antibody–VEGF binding. However, all these therapeutic regimens require frequent administrations of large doses of the inhibitors (Holash et al., 2002; Ferrara et al., 2004). In a previous study, we demonstrated that genetic delivery of monoclonal antibody A.4.6.1 suppressed tumor growth in a human tumor xenograft model after a single administration, suggesting that genetic delivery of anti-VEGF antibodies may be a strategy to further increase antibody half-life and consequent bioavailability (Watanabe et al., 2008).
Treatment of high-permeability pulmonary edema with bevacizumab
The effects of bevacizumab in inhibiting angiogenesis and tumor growth are striking and suggest that inhibition of other VEGF-dependent processes with bevacizumab would be similarly effective. Although there have been no published studies demonstrating the utility of bevacizumab as a therapy for high-permeability pulmonary edema in humans, there are anecdotal reports that bevacizumab is effective in treating pleural effusion (Badros et al., 2005; Pichelmayer et al., 2005; Hoyer et al., 2007). The connection between VEGF and the establishment of high-permeability pulmonary edema suggests that anti-VEGF antibodies are a viable therapeutic strategy for this condition. Keeping in mind that VEGF has multiple functions, including a role in maintaining alveolar structure and function and in repair after injury (Mura et al., 2004; Voelkel et al., 2006; Kosmidou et al., 2008), transient administration of anti-VEGF to treat high-permeability pulmonary edema will have to be assessed from a safety viewpoint. With this caveat, the data obtained from the current study demonstrate the efficacy of genetic delivery of an anti-VEGF antibody in suppressing VEGF-induced high-permeability pulmonary edema in a mouse model and suggest that bevacizumab, either directly administered or genetically delivered, is a novel strategy for the prevention of high-permeability pulmonary edema.
Acknowledgments
The authors thank Neil R. Hackett and Jasen Murray for help with quantitative TaqMan real-time PCR, Robert J. Kaner and Bishnu De for helpful discussions, and Nahla Mohamed for help in preparing this manuscript. These studies were supported, in part, by U01 HL66952 and P01 HL59312.
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Baca M. Wells J.A. Anti-VEGF antibodies. 1997. Genentech, Inc. U.S. Patent 908469 [6884879].
- Baca M. Presta L.G. O'Connor S.J. Wells J.A. Antibody humanization using monovalent phage display. J. Biol. Chem. 1997;272:10678–10684. doi: 10.1074/jbc.272.16.10678. [DOI] [PubMed] [Google Scholar]
- Badros A. Porter N. Zimrin A. Bevacizumab therapy for POEMS syndrome. Blood. 2005;106:1135. doi: 10.1182/blood-2005-03-0910. [DOI] [PubMed] [Google Scholar]
- Bates D.O. Lodwick D. Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6:83–96. [PubMed] [Google Scholar]
- Becker P.M. Alcasabas A. Yu A.Y. Semenza G.L. Bunton T.E. Oxygen-independent upregulation of vascular endothelial growth factor and vascular barrier dysfunction during ventilated pulmonary ischemia in isolated ferret lungs. Am. J. Respir. Cell Mol. Biol. 2000;22:272–279. doi: 10.1165/ajrcmb.22.3.3814. [DOI] [PubMed] [Google Scholar]
- Bhandari V. Choo-Wing R. Chapoval S.P. Lee C.G. Tang C. Kim Y.K. Ma B. Baluk P. Lin M.I. McDonald D.M. Homer R.J. Sessa W.C. Elias J.A. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc. Natl. Acad. Sci. U.S.A. 2006;103:11021–11026. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V. Choo-Wing R. Lee C.G. Yusuf K. Nedrelow J.H. Ambalavanan N. Malkus H. Homer R.J. Elias J.A. Developmental regulation of NO-mediated VEGF-induced effects in the lung. Am. J. Respir. Cell Mol. Biol. 2008;39:420–430. doi: 10.1165/rcmb.2007-0024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen E.C. VEGF in biological control. J. Cell. Biochem. 2007;102:1358–1367. doi: 10.1002/jcb.21579. [DOI] [PubMed] [Google Scholar]
- Carpenter T. Schomberg S. Steudel W. Ozimek J. Colvin K. Stenmark K. Ivy D.D. Endothelin B receptor deficiency predisposes to pulmonary edema formation via increased lung vascular endothelial cell growth factor expression. Circ. Res. 2003;93:456–463. doi: 10.1161/01.RES.0000090994.15442.42. [DOI] [PubMed] [Google Scholar]
- Carpenter T.C. Schomberg S. Stenmark K.R. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L1075–L1082. doi: 10.1152/ajplung.00251.2005. [DOI] [PubMed] [Google Scholar]
- Choi W.I. Quinn D.A. Park K.M. Moufarrej R.K. Jafari B. Syrkina O. Bonventre J.V. Hales C.A. Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 2003;167:1627–1632. doi: 10.1164/rccm.200210-1216OC. [DOI] [PubMed] [Google Scholar]
- De B.P. Hackett N.R. Crystal R.G. Boyer J.L. Rapid/sustained anti-anthrax passive immunity mediated by co-administration of Ad/AAV. Mol. Ther. 2008;16:203–209. doi: 10.1038/sj.mt.6300344. [DOI] [PubMed] [Google Scholar]
- Dvorak H.F. Discovery of vascular permeability factor (VPF) Exp. Cell Res. 2006;312:522–526. doi: 10.1016/j.yexcr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Dvorak H.F. Brown L.F. Detmar M. Dvorak A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Esser S. Wolburg K. Wolburg H. Breier G. Kurzchalia T. Risau W. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J. Cell Biol. 1998;140:947–959. doi: 10.1083/jcb.140.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Vascular endothelial growth factor: Basic science and clinical progress. Endocr. Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Gerber H.P. Lecouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Hillan K.J. Gerber H.P. Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- Ferrara N. Hillan K.J. Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem. Biophys. Res. Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Fox W.D. Higgins B. Maiese K.M. Drobnjak M. Cordon-Cardo C. Scher H.I. Agus D.B. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin. Cancer Res. 2002;8:3226–3231. [PubMed] [Google Scholar]
- Fraser R.S. Colman N. Muller N.L. Pare P.D. Pulmonary edema. In: Fraser R.S., editor; Coleman N., editor; Muller N.L., editor; Pare P.D., editor. Fraser and Pare's Diagnosis of Diseases of the Chest. W.B. Saunders; Philadelphia: 1999. pp. 1946–2017. [Google Scholar]
- Fukumura D. Gohongi T. Kadambi A. Izumi Y. Ang J. Yun C.O. Buerk D.G. Huang P.L. Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H.P. Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- Godzich M. Hodnett M. Frank J.A. Su G. Pespeni M. Angel A. Howard M.B. Matthay M.A. Pittet J.F. Activation of the stress protein response prevents the development of pulmonary edema by inhibiting VEGF cell signaling in a model of lung ischemia–reperfusion injury in rats. FASEB J. 2006;20:1519–1521. doi: 10.1096/fj.05-4708fje. [DOI] [PubMed] [Google Scholar]
- Gorin A.B. Stewart P.A. Differential permeability of endothelial and epithelial barriers to albumin flux. J. Appl. Physiol. 1979;47:1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- Gropper M.A. Wiener-Kronish J. The epithelium in acute lung injury/acute respiratory distress syndrome. Curr. Opin. Crit. Care. 2008;14:11–15. doi: 10.1097/MCC.0b013e3282f417a0. [DOI] [PubMed] [Google Scholar]
- Gurkan O.U. O'Donnell C. Brower R. Ruckdeschel E. Becker P.M. Differential effects of mechanical ventilatory strategy on lung injury and systemic organ inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2003;285:L710–L718. doi: 10.1152/ajplung.00044.2003. [DOI] [PubMed] [Google Scholar]
- Guyton A.C. Lindsey A.W. Effect of elevated left atrial pressure and decreased plasma protein concentration on the development of pulmonary edema. Circ. Res. 1959;7:649–657. doi: 10.1161/01.res.7.4.649. [DOI] [PubMed] [Google Scholar]
- Hackett N.R. Crystal R.G. Adenovirus vectors for gene therapy. In: Lasic D., editor; Templeton N.S., editor. Gene Therapy: Therapeutic Mechanisms and Strategies. Third Edition. Marcel Dekker; New York: 2008. pp. 39–68. [Google Scholar]
- Hersh J. Crystal R.G. Bewig B. Modulation of gene expression after replication-deficient, recombinant adenovirus-mediated gene transfer by the product of a second adenovirus vector. Gene Ther. 1995;2:124–131. [PubMed] [Google Scholar]
- Hippenstiel S. Krull M. Ikemann A. Risau W. Clauss M. Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am. J. Physiol. 1998;274:L678–L684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- Holash J. Davis S. Papadopoulos N. Croll S.D. Ho L. Russell M. Boland P. Leidich R. Hylton D. Burova E. Ioffe E. Huang T. Radziejewski C. Bailey K. Fandl J.P. Daly T. Wiegand S.J. Yancopoulos G.D. Rudge J.S. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer R.J. Leung N. Witzig T.E. Lacy M.Q. Treatment of diuretic refractory pleural effusions with bevacizumab in four patients with primary systemic amyloidosis. Am. J. Hematol. 2007;82:409–413. doi: 10.1002/ajh.20858. [DOI] [PubMed] [Google Scholar]
- Hurwitz H. Fehrenbacher L. Novotny W. Cartwright T. Hainsworth J. Heim W. Berlin J. Baron A. Griffing S. Holmgren E. Ferrara N. Fyfe G. Rogers B. Ross R. Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Kaner R.J. Crystal R.G. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol. Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- Kaner R.J. Crystal R.G. Pathogenesis of high altitude pulmonary edema: Does alveolar epithelial lining fluid vascular endothelial growth factor exacerbate capillary leak? High Alt. Med. Biol. 2004;5:399–409. doi: 10.1089/ham.2004.5.399. [DOI] [PubMed] [Google Scholar]
- Kaner R.J. Ladetto J.V. Singh R. Fukuda N. Matthay M.A. Crystal R.G. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 2000;22:657–664. doi: 10.1165/ajrcmb.22.6.3779. [DOI] [PubMed] [Google Scholar]
- Karmpaliotis D. Kosmidou I. Ingenito E.P. Hong K. Malhotra A. Sunday M.E. Haley K.J. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;283:L585–L595. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]
- Kim K.J. Li B. Houck K. Winer J. Ferrara N. The vascular endothelial growth factor proteins: Identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7:53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- Kosmidou I. Karmpaliotis D. Kirtane A.J. Barron H.V. Gibson C.M. Vascular endothelial growth factors in pulmonary edema: An update. J. Thromb. Thrombolysis. 2008;25:259–264. doi: 10.1007/s11239-007-0062-4. [DOI] [PubMed] [Google Scholar]
- Kowanetz M. Ferrara N. Vascular endothelial growth factor signaling pathways: Therapeutic perspective. Clin. Cancer Res. 2006;12:5018–5022. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- Kunig A.M. Balasubramaniam V. Markham N.E. Seedorf G. Gien J. Abman S.H. Recombinant human VEGF treatment transiently increases lung edema but enhances lung structure after neonatal hyperoxia. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L1068–L1078. doi: 10.1152/ajplung.00093.2006. [DOI] [PubMed] [Google Scholar]
- Kuo C.J. Farnebo F. Yu E.Y. Christofferson R. Swearingen R.A. Carter R. Von Recum H.A. Yuan J. Kamihara J. Flynn E. D'Amato R. Folkman J. Mulligan R.C. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaver S.K. Evans T.W. Acute respiratory distress syndrome. BMJ. 2007;335:389–394. doi: 10.1136/bmj.39293.624699.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.G. Link H. Baluk P. Homer R.J. Chapoval S. Bhandari V. Kang M.J. Cohn L. Kim Y.K. McDonald D.M. Elias J.A. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat. Med. 2004;10:1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangeli A. Danel C. Rosenfeld M.A. Stratford-Perricaudet L. Perricaudet M. Pavirani A. Lecocq J.P. Crystal R.G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J. Clin. Invest. 1993;91:225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T. Claesson-Welsh L. VEGF receptor signal transduction. Sci. STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Matthay M.A. Martin T.A. Pulmonary edema and acute lung injury. In: Mason R., editor; Broaddus V., editor; Murray J., editor; Nadel J., editor. Murray and Nadel's Textbook of Respiratory Medicine. Elsevier Saunders; Philadelphia: 2005. pp. 1502–1543. [Google Scholar]
- Matthay M.A. Folkesson H.G. Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol. Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- Miller K.D. Chap L.I. Holmes F.A. Cobleigh M.A. Marcom P.K. Fehrenbacher L. Dickler M. Overmoyer B.A. Reimann J.D. Sing A.P. Langmuir V. Rugo H.S. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J. Clin. Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- Mittereder N. March K.L. Trapnell B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J.S. Tsang J. Evans K.G. Mullen J.B. Burns A.R. Walker D.C. Wiggs B. Hogg J.C. Alveolar epithelial damage: A critical difference between high pressure and oleic acid-induced low pressure pulmonary edema. J. Clin. Invest. 1986;77:1786–1796. doi: 10.1172/JCI112503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlhauser J. Merrill M.J. Pili R. Maeda H. Bacic M. Bewig B. Passaniti A. Edwards N.A. Crystal R.G. Capogrossi M.C. VEGF165 expressed by a replication-deficient recombinant adenovirus vector induces angiogenesis in vivo. Circ. Res. 1995;77:1077–1086. doi: 10.1161/01.res.77.6.1077. [DOI] [PubMed] [Google Scholar]
- Mura M. Dos Santos C.C. Stewart D. Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J. Appl. Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]
- Olsson A.K. Dimberg A. Kreuger J. Claesson-Welsh L. VEGF receptor signalling: In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- Pichelmayer O. Zielinski C. Raderer M. Response of a nonmalignant pleural effusion to bevacizumab. N. Engl. J. Med. 2005;353:740–741. doi: 10.1056/NEJM200508183530722. [DOI] [PubMed] [Google Scholar]
- Plikaytis B.D. Turner S.H. Gheesling L.L. Carlone G.M. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1991;29:1439–1446. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta L.G. Chen H. O'Connor S.J. Chisholm V. Meng Y.G. Krummen L. Winkler M. Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- Proescholdt M.A. Heiss J.D. Walbridge S. Muhlhauser J. Capogrossi M.C. Oldfield E.H. Merrill M.J. Vascular endothelial growth factor (VEGF) modulates vascular permeability and inflammation in rat brain. J. Neuropathol. Exp. Neurol. 1999;58:613–627. doi: 10.1097/00005072-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Roberts W.G. Palade G.E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Siegfried W. Yoshimura K. Yoneyama K. Fukayama M. Stier LE. Paakko P.K. Gilardi P. Stratford-Perricaudet L.D. Perricaudet M. Jallat S. Pavirani A. Lecocq J.-P. Crystal R.G. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M.A. Yoshimura K. Trapnell B.C. Yoneyama K. Rosenthal E.R. Dalemans W. Fukayama M. Bargon J. Stier L.E. Stratford-Perricaudet L. Perricaudet M. Guggino W.B. Pavirani A. Lecocq J.-P. Crystal R.G. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Rosenstein J.M. Krum J.M. New roles for VEGF in nervous tissue: Beyond blood vessels. Exp. Neurol. 2004;187:246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Sandler A. Gray R. Perry M.C. Brahmer J. Schiller J.H. Dowlati A. Lilenbaum R. Johnson D.H. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Schoch H.J. Fischer S. Marti H.H. Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain. 2002;125:2549–2557. doi: 10.1093/brain/awf257. [DOI] [PubMed] [Google Scholar]
- Senger D.R. Galli S.J. Dvorak A.M. Perruzzi C.A. Harvey V.S. Dvorak H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Staub N.C. Pulmonary edema. Physiol. Rev. 1974;54:678–811. doi: 10.1152/physrev.1974.54.3.678. [DOI] [PubMed] [Google Scholar]
- Sweeney C.J. Miller K.D. Sissons S.E. Nozaki S. Heilman D.K. Shen J. Sledge G.W., Jr. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–3372. [PubMed] [Google Scholar]
- Thickett D.R. Armstrong L. Christie S.J. Millar A.B. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2001;164:1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- Tischer E. Mitchell R. Hartman T. Silva M. Gospodarowicz D. Fiddes J.C. Abraham J.A. The human gene for vascular endothelial growth factor: Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- Turner C.R. Zuczek S. Knudsen D.J. Wheeldon E.B. Microwave fixation of the lung. Stain Technol. 1990;65:95–101. doi: 10.3109/10520299009108063. [DOI] [PubMed] [Google Scholar]
- Voelkel N.F. Vandivier R.W. Tuder R.M. Vascular endothelial growth factor in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Boyer J.L. Hackett N.R. Qiu J. Crystal R.G. Genetic delivery of the murine equivalent of bevacizumab (avastin), an anti-vascular endothelial growth factor monoclonal antibody, to suppress growth of human tumors in immunodeficient mice. Hum. Gene Ther. 2008;19:300–310. doi: 10.1089/hum.2007.109. [DOI] [PubMed] [Google Scholar]
- Wheeler A.P. Bernard G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- Yang J.C. Haworth L. Sherry R.M. Hwu P. Schwartzentruber D.J. Topalian S.L. Steinberg S.M. Chen H.X. Rosenberg S.A. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]