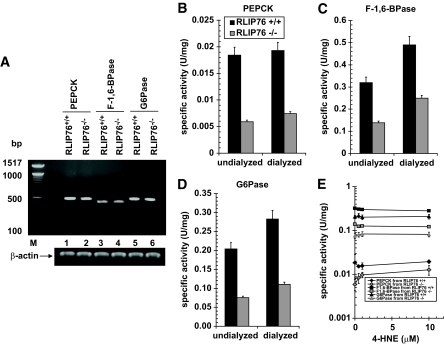
FIG. 6.
The activity of gluconeogenesis enzymes. The activity of PEPCK (B), F-1,6-BPase (C), and G6Pase (D) was measured in undialyzed and dialyzed liver homogenates of RLIP76+/+ and RLIP76−/− animals. The effect of 4-HNE was also determined on the activity for all three important enzymes of gluconeogenesis (E). The enzyme PEPCK catalyzes the conversion of phosphoenolpyruvate to fructose1,6-biphosphate in a series of steps involving oxidation of NADH to NAD. In this assay, the loss of NADH was determined spectrophotometrically by measuring absorbance at 340 nm, based on the method of Opie and Newsholme (29). For F-1,6-BPase activity, a spectrophotometric-coupled enzyme assay was used based on the method of Taketa and Pogell (30). F-1,6-BPase activity was coupled with phosphoglucose isomerase and NADP-dependent glucose 6-phosphate dehydrogenase, and NADPH formation was measured at 340 nm. G6Pase activity was determined spectrophotometrically using the method of Gierow and Jergil (31). The method is based on a coupled enzyme reaction in which glucose formed is reacted with glucose oxidase and peroxidase, and the quinoneimine formed is a colored product and its formation can be followed spectrophotometrically at 510 nm. The expression of all three enzymes was determined by RT-PCR using their gene-specific primers (A). Either dialyzed or undialyzed, P < 0.01, when compared with RLIP76+/+ vs. RLIP76−/−. RLIP76+/+ or RLIP76−/−, P < 0.07, when compared with undialyzed vs. dialyzed. Means ± SD for three separate experiments, each in triplicate, are shown; n = 9.