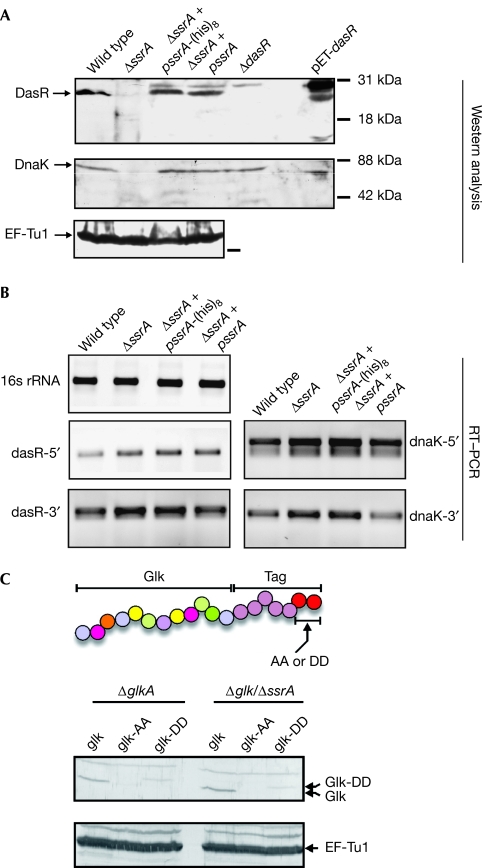
Figure 2.
Translation, but not transcription, of dasR and dnaK depends on tmRNA tagging. (A) Proteins were analysed by western blotting using DasR, DnaK or EF-Tu1 antibodies. Escherichia coli overexpressing DasR (pET-dasR) was used as a control (E. coli DnaK is smaller than that of Streptomyces coelicolor). (B) RT–PCR of total RNA from the different strains, demonstrating that transcription of the dasR and dnaK genes is not affected in ssrA mutants. (C) Engineered carboxy-terminally tagged glucose kinase is degraded in a canonical manner. Protein extracts from transformants expressing wild-type Glk, GlkAA or GlkDD were separated by 12% SDS–PAGE and immunodetected with Glk antibodies. As a control, EF-Tu1 antibodies were used. The experiment demonstrates that tmRNA-tagged proteins are efficiently degraded in Streptomyces. A schematic representation of the translational fusion of wild-type tmRNA tag (AA; giving GlkAA) or a mutated tmRNA tag (DD; giving GlkDD) to glucose kinase is shown. DasR, development of aerial mycelium and spores regulator; EF, elongation factor; Glk, glucose kinase; RT–PCR, reverse transcriptase PCR; SDS–PAGE, sodium dodecyl sulphate–polyacrylamide gel electrophoresis; tmRNA, transfer–messenger RNA.