Abstract
In a non-viral gene delivery system, localization of a plasmid DNA in the nucleus is a prerequisite for expression of a desired therapeutic protein encoded in the plasmid DNA. In this study, a reducible polymer-based gene delivery system for improved intracellular trafficking and nuclear translocation of plasmid DNA is introduced. The system is consisted of two components, a plasmid DNA having repeated biding sequence for a karyophilic protein, NFκB, and a reducible polymer. A reducible poly(amido ethylenimine), poly(TETA-CBA), was synthesized by a Michael-type addition polymerization between cystamine bisacrylamide and triethyl tetramine. The polymer forming tight complexes with plasmid DNA could be degraded in the reductive cytosol to release the plasmid DNA. The triggered release mechanism in the cytosol could facilitate the interaction between cytosolic NFκB and the plasmid DNA having repeated NFκB biding motif. Upon activation of NFκB by interleukin-1β (IL-1β), the most of plasmid distributed in the cytoplasm was localized within the nucleus, resulting in significantly higher gene transfection efficiency than controls with non-degradable PEI. The current study suggests an alternative way of improving transfection efficiency by taking advantage of endogenous transport machinery for intracellular trafficking and nuclear translocation of a plasmid DNA.
Keywords: Non-viral gene delivery, reducible polymer, intracellular trafficking, nuclear localization
INTRODUCTION
Over the last decades, a lot of non-viral gene delivery systems based on synthetic materials have been developed to replace virus-originated gene carriers that are often questioned for safety issues (1, 2). However, there is still a room for improving transfection efficiency of non-viral methods. Synthetic carriers should overcome a number of extracellular and intracellular barriers to deliver plasmid DNA to the cell nucleus where a gene transcription occurs. Among the challenging barriers, the dissociation of plasmid DNA from a carrier complex, the transport of plasmid DNA to the peri-nuclear space in the cytoplasm, and the passage of plasmid DNA across the nuclear membrane are considered as rate-limiting steps in a polymer-based gene delivery carrier (3, 4). Although efficient intracellular trafficking and nuclear translocation of plasmid DNA is essential to achieve desirable transfection efficiency, the delivery mechanism by a non-viral gene carrier has not been clearly understood. One possible mechanism is the nucleus localization of plasmid DNA by disruption of the nuclear envelope during mitotic cell division (5). Active nuclear transport of plasmid DNA by employing nuclear localization signal (NLS) was previously suggested (6), but the effectiveness of NLS in transfection still seems to be controversial (7). Recent studies showed that plasmids containing a specific sequence recognized by a nuclear protein (nuclear factor κB, NFκB) could improve transfection possibly due to nuclear-protein guided intracellular trafficking and active nuclear translocation of the plasmid DNA (8-10). However, in this case, the release of plasmid DNA from carrier/DNA complexes is a prerequisite for the interaction of the nuclear protein to plasmid DNA that may lead to the guided trafficking and nuclear transport of plasmid DNA.
We previously demonstrated reducible poly(amido ethylenimine)s successfully carry out unpacking of complexes owing to a reductive degradation of the polymer backbone in the cytoplasm, leading to enhanced transfection efficiency (11, 12). In this study, we used a plasmid DNA containing repeated NFκB binding sites in conjunction with the reducible poly(amido ethylenimine) to improve cytosolic release of plasmid DNA. It was hypothesized that the triggered release of the plasmid DNA with the NFκB binding sites in the reductive cytoplasm would facilitate the access of NFκB to the plasmid DNA to improve nuclear translocation, which may consequently lead to enhancement of transfection efficiency.
MATERIALS AND METHODS
Materials
For polymer synthesis, triethylenetetramine (TETA) and cystamine bisacrylamide (CBA) were purchased from Sigma-Aldrich (St. Louis, MO) and Polysciences (Warrington, PA), respectively, and were used without further purification. Branched polyethylenimine (bPEI, Mw 25,000), ethidium bromide, buthionine sulfoxamine (BSO), dithiothreitol (DTT), and (3-(4,5-dimethylthyazolyl-2)-2,5-diphenyl tetrazolium bromide) (MTT) was obtained from Sigma-Aldrich (St. Louis, MO). All cell culture products including Fetal bovine serum (FBS) and Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Invitrogen (Carlsbad, CA). Mouse interleukin-1β (IL-1β) and a protease inhibitor cocktail containing aprotinin, bestatin, leupeptin, E-64, and pepstatin A were obtained from Sigma-Aldrich (St. Louis, MO).
Synthesis of a reducible TETA-CBA copolymer
Reducible TETA-CBA copolymer was synthesized by Michael type addition polymerization as described previously (11). Briefly, the addition polymerization between TETA (449 mg, 3.1 mmol) and CBA (800 mg, 3.1 mmol) was performed in 10 % aqueous methanol at 50 °C for 16h. The reaction was terminated by adding excess amount of the amine donor (TETA). The product was purified by ultrafiltration (MWCO 1,000) and lyophilized. The chemical composition of the synthesized polymer was estimated by using 1H-NMR. The polymer molecular weight (Mw) was 3,350, as determined by gel permeation chromatography (GPC).
Plasmid DNA
A plasmid DNA (pSIGLP1NFκB) containing an expression cassette of GLP-1 and five direct repeats of NFκB binding motif (5′-GGGGACTTTCC-3′) was constructed as described in a previous report (9). The repeated NFκB binding sites was inserted after SV40 polyadenylation site (Figure 1). Plasmid DNA was purified from E.coli by an alkaline lysis method using a silica-based anion exchange column (Qiagen, Valencia, CA).
Figure 1.
A schematic illustration of a gene delivery system based on a reducible polymer and a plasmid that can interact with cytosolic karyophilic protein, NFκB.
Electrophoretic mobility shift assays
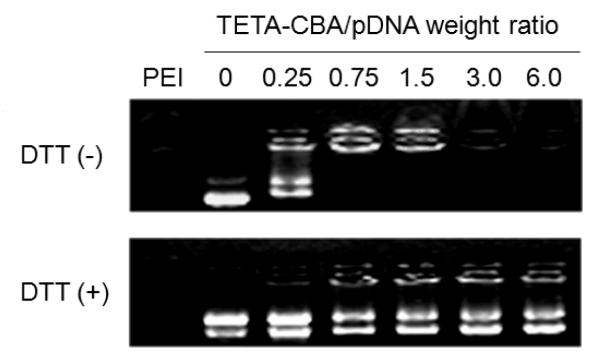
Varying amount of poly(TETA/CBA) and a fixed amount of plasmid DNA (300 ng) were separately diluted in PBS. The diluted polymer and DNA solutions were combined at varying weight ratios and mixed with gentle vortexing to form complexes. Dithiothreitol (DTT, 2.5 mM) was added to one of the test groups to observe the release of plasmid DNA by a reductive degradation of the polymer. The complexes were allowed to be stabilized for 30 min at an ambient temperature and loaded onto 0.8 % agarose gel. Electrophoresis was performed with a current of 120 V for 20 min in TAE buffer solution (40 mM Tris/HCl, 1 % (v/v) acetic acid, 1 mM EDTA). The retardation of the complexes was visualized by using an image analyzer equipped with UV transilluminator (GelDoc, BioRad, Hercules, CA) after ethidium bromide staining.
Cell culture and transfection
Mouse fibroblast cells (NIH3T3) was cultured in DMEM supplemented with 10 % FBS and maintained at 37 °C in a humidified 5 % CO2 atmosphere. Before transfection, the cells were trypsinized and 105 cells per well were plated in a six-well plate (35mm diameter). The cells were then incubated at 37 °C in a humidified 5 % CO2 atmosphere for 24 h.
Before transfection, the plasmid DNA (2 μg) and the desired amount of either TETA-CBA (DNA:polymer = 10:1 w/w) or bPEI 25kDa (DNA:polymer = 0.75:1 w/w) were separately diluted in PBS and mixed. The mixture was incubated to form complexes for 15 min at room temperature and added to the cells. The cell culture medium was replaced with serum-free medium prior to the addition of the complexes. After 3 h incubation at 37 °C, the medium was removed and supplemented with a fresh cell culture medium containing 10 % FBS and a protease inhibitor cocktail. For the activation and nuclear translocation of cytoplasmic NFκB, IL-1β (20 ng/ml) was added to the medium. The concentration of GLP-1 was determined by ELISA (Linco Research, St. Charles, MO), as previously described (13).
Flow cytometry
To determine cellular association of the transfection complexes formed from a desired polymer (either TETA-CBA or PEI), the transfected cells were trypsinized and washed three times with cold PBS, and fixed by 1 % paraformaldehyde at 4 °C before flow cytometric analysis (FACS Caliber, Becton-Dickinson, Mount View, CA). At least 1×104 events were analyzed to generate each diagram.
Confocal microscopy
The cells were grown in glass-bottoed dishes (Glass Bottom Microwells; MatTek Corp., Ashland, MA). The plasmid DNA was fluorescently labeled with Cy3 by using Label-IT kit (Mirus Bio, Madison, WI). The transfection and NFκB activation were carried out as described above. The transfected cells were incubated for additional 2 hours in the absence and presence of 20 ng/ml of IL-1β, prior to the observation. The cells were then washed five times with cold PBS, fixed by submerging the cells in 1 % paraformaldehyde at 4 °C for 30 min, and washed three times with cold PBS. The subcellular localization of the complexes was visualized by a laser scanning confocal fluorescence microscopy using Olympus Fluoview FV300 microscope (Melville, NY). An oil immersion objective lens was used for the epidetection configuration. Nuclear localization of plasmid DNA was quantified by relating fluorescence intensities from two arbitrarily defined regions of interest (ROIs): the whole cell (FLcell) and the nucleus (FLnucleus). Fluorescence intensity from each ROI was separately analyzed by using ImageJ v1.42 software (http://rsb.info.nih.gov/ij). Percent fluorescence in the selected nucleus region (FLnucleus / FLcell × 100) was calculated and presented as mean % nuclear localization ± SEM (n=50).
RESULTS AND DISCUSSION
Viruses have evolved to transfer their genetic information to host cells for the production of progeny. For the efficient delivery of its genome to host nucleus, a virus contains multiple functional components that facilitates cellular entry, endosomal escape, the dissociation of genetic material from capsid protein complex, and nuclear transfer by the aid of host nuclear localization mechanism. It has long been a challenging problem to develop a synthetic carrier that mimics the viral infection machinery (14). Gene therapy based on a plasmid DNA encoding a desired therapeutic gene depends largely on a successful entry of plasmid DNA into the nucleus, independent of a type of gene delivery method. After cellular entry and endosomal escape, a carrier/plasmid DNA complex or a plasmid DNA released from the complex should move toward the peri-nuclear space where nuclear translocation of a plasmid DNA occurs. However, the nano-particular complex or a plasmid DNA with several millions of molecular weight can hardly pass through a highly viscous medium of the cytoplasm filled with an extensive network of cytoskeletons, proteins, mRNAs, and various subcellular organelles (15, 16). In addition, the passage of macromolecules through the nuclear envelope is also restricted. The nuclear pore complex only allows the passage of biological macromolecules with molecular weight of less than 60 kDa unless they have a signal peptide for nuclear localization (NLS), which can be recognized by nuclear importins, carrier proteins for nuclear localization of various karyophilic proteins. Therefore, we designed a virus-mimicking gene delivery system that can use an endogenous nuclear transport mechanism by using a reducible polymer that is degradable in the cytoplasm and a plasmid DNA containing a repeated binding motif for NFκB, an endogenous transcription factor. The plasmid DNA was designed to use the transcription factor as an intracellular carrier for active nuclear translocation of a plasmid DNA. A ubiquitously expressed transcription factor, NFκB, mainly exists as an inactive heterodimer with IκB protein in the cytoplasm (17). When the cell is stimulated by a specific signal, IκB is phosphorylated and rapidly degraded. This results in the exposure of NLS motif, leading to rapid nuclear translocation of NFκB (18). It was previously reported that a plasmid containing NFκB binding sequence demonstrated enhanced cytoplasmic mobility and nuclear translocation of the plasmid DNA (8), leading to enhanced transfection efficiency (9, 19). Since the interaction between the plasmid DNA and NFκB is a prerequisite to achieve efficient karyophilic protein-guided cytosolic trafficking and nuclear translocation, unpackaging of the plasmid DNA/polymer complexes should be occurred soon after the endosomal escape of the complexes. An endosomal escape and the cytosolic unpacking of the complexes could be facilitated by using a reducible polymer, TETA-CBA.
A reducible TETA-CBA copolymer was synthesized by Michael-addition polymerization between amine donor (TETA) and bisacrylate (CBA), as describe previously (11). The disulfide linkages in TETA-CBA are expected to be cleaved into amidoamine monomers in the reductive cytoplasm. The proton-buffering and reductive degradation properties of the copolymer were studied in a previously published result (11, 12). The cationic copolymer interacts with plasmid DNA to form tight complexes for efficient gene delivery to cells (11, 20). Once localized inside the cells, the backbone of TETA-CBA may be degraded by the action of intracellular reduced glutathione, resulting in the cytosolic release of the plasmid DNA. The released plasmid DNA containing the repeated NFκB motif could then bind to the DNA binding domain of cytosolic NFκB without interference of the cationic carrier. The NFκB bound to the plasmid could act as an intracellular vehicle for the plasmid DNA. Stimulation of the cells with a cytokine such as IL-1β causes the degradation of IκB unit and the exposure of an NLS moiety of NFκB, leading to intracellular trafficking of the NFκB-plasmid DNA to the perinuclear space and concurrent transfer of the NFκB-plasmid DNA into the nucleus. The hypothetical intracellular delivery mechanism based on a reducible polycation and a plasmid DNA with a karyophilic protein biding site is illustrated in Figure 1.
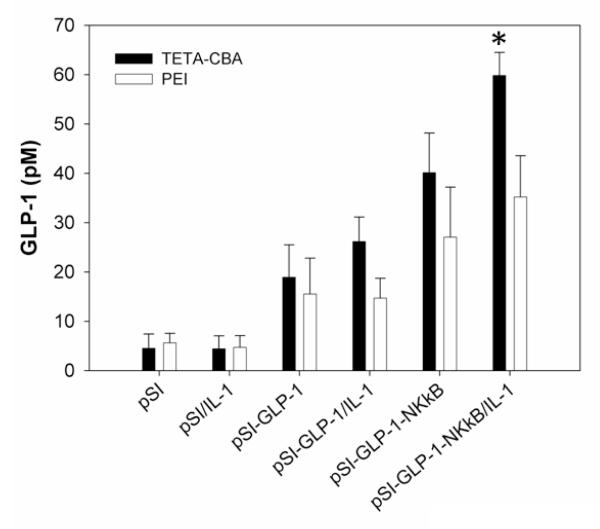
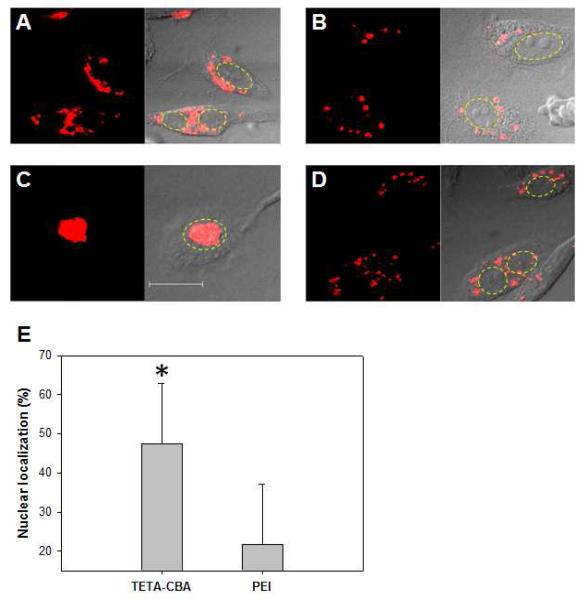
A plasmid DNA (pSIGLP1NFκB) encoding glucagon-like peptide-1 (GLP-1) was previously constructed (9) and used to evaluate gene transfection efficiency. The electrostatic interaction between the plasmid DNA and TETA-CBA polymer could form tight complexes, which could be readily degraded to release the plasmid DNA in the presence of a reducing force (Figure 2). The degradation of the polymer in a reductive environment agrees well with previous results (11). The pSIGLP1NFκB/TETA-CBA formulation demonstrated only a slightly higher production of GLP-1 in mouse fibroblast cells (NIH3T3) than a formulation with a non degradable control polymer, PEI 25 kDa (Figure 3). The addition of IL-1β, which can initiate the dissociation of IκB from NFκB heterodimer and the nuclear translocation of NFκB (18), to the cells treated with the pSIGLP1NFκB/TETA-CBA formulation resulted in a significant increase in GLP-1 expression. However, the control formulation (pSIGLP1NFκB/PEI) failed to show a significant increase in GLP-1 expression after the IL-1β stimulation. A cellular association profile of the transfection complexes from either TETA-CBA or PEI-based formulation was not significantly different from each other (Figure 4). The results suggest that the improved transfection efficiency may be due to the NFκB-guided intracellular trafficking and active nuclear translocation of the plasmid DNA. To test the hypothetical suggestion, the subcellular localization of plasmid DNA after transfection was observed using a confocal-laser scanning microscopy. The Cy-3 labeled plasmid DNA formulated with TETA-CBA was distributed over the cytoplasm 5 h after transfection (Figure 5A). However, most of the fluorescently-labeled plasmid DNA was localized in the cytoplasmic space, rather than in the nucleus, suggesting that the DNA dissociated from the polymer/DNA complex could not penetrate the nucleus membrane via passive diffusion. The uneven distribution of the plasmid DNA in the cytoplasm may be due to restricted movement of high molecular weight plasmid DNA in the highly viscous mesh-like network of the cytoplasm. The cytosolic reductive release of genetic materials such as plasmid DNA and siRNA could also be observed in previous observations with reducible polymeric carriers (21, 22). In contrast to the TETA-CBA formulation, PEI formulation seemed to exist as complexes or larger aggregates in a specific location, suggesting complexes formed from PEI and plasmid DNA may not be readily dissociated in the cytoplasm (Figure 5B). When stimulated by IL-1β, nuclear accumulation of the fluorescently labeled plasmid DNA (pSIGLP1NFκB) could be observed in the cells treated with the pSIGLP1NFκB/TETA-CBA formulation (Figure 5C). However, no significant movement of the plasmid DNA to the nucleus region was detected in the cells transfected with the pSIGLP1NFκB/PEI formulation, even after IL-1β treatment (Figure 5D). The improved nuclear localization was quantitatively measured by using image analysis software (ImageJ). Nuclear localization of the fluorescently labeled plasmid DNA was obtained by relating fluorescence intensities from two arbitrarily defined regions of interest (ROIs): the whole cell (FLcell) and the nucleus (FLnucleus). After stimulation with IL-1β, the pSIGLP1NFκB/TETA-CBA formulation showed a significantly higher accumulation of the plasmid DNA in the nucleus region compared to PEI formulation. In conjunction with the qualitative observation with confocal microscopy, the data suggests that the reductive degradation of the reducible polymer and release of the plasmid DNA in the cytoplasm would play important role in the NFκB-mediated cytosolic trafficking and the nuclear import of the plasmid DNA by facilitating the interaction between NFκB and the binding motif in the plasmid DNA.
Figure 2.
Electrophoretic mobility shift assays of poly(TETA/CBA)/plasmid DNA complexes at various polymer/siRNA weight ratios. Dithiothreitol (DTT, 2.5 mM) was added to generate a reductive environment to trigger the release of plasmid DNA from the complexes.
Figure 3.
Transfection efficiencies of various formulations based on either TETA-CBA or PEI. IL-1β was added to activate nuclear translocation of NFκB. The expression level of secreted GLP-1 was determined by ELISA and given as the mean ± S.D. (n=3). *p<0.01 compare to the PEI-based control.
Figure 4.
Cellular association of pSIGLP1NFκB/TETA-CBA and pSIGLP1NFκB/PEI complexes, analyzed by flow cytometry.
Figure 5.
Subcellular localization of Cy3-labeled pSIGLP1NFκB formulated with either TETA-CBA (A, C) or PEI (B, D) after transfection in NIH3T3 cells. Prior to the observation, the cells were incubated for additional 2 h in the absence (A, B) and presence (C, D) of IL-1β stimulation. Dashed line indicates the nucleus. (E) Nuclear association of plasmid DNA after IL-1β stimulation was determined by relating two arbitrarily defined regions of interest (ROIs: ROI1=the whole cell, ROI2=the nucleus) and given as percent nuclear localization ± S.D. (n=50). *p<0.05 compare to the PEI-based control.
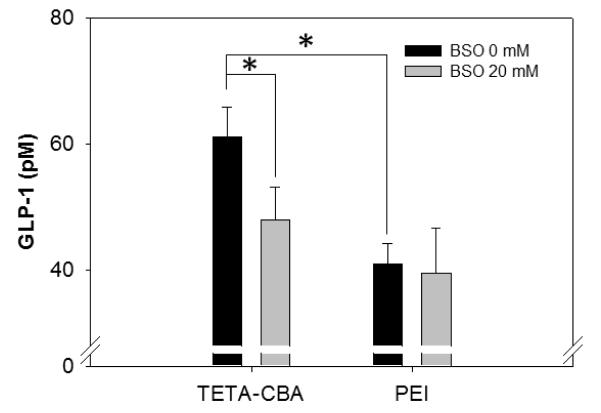
The role of cytosolic reductive degradation of the TETA-CBA in NFκB-mediated nuclear translocation of pSIGLP1NFκB was further studied by using DL-buthionine sulfoxamine (BSO), which effectively reduces the level of intracellular reduced glutathione (23). Pre-treatment of the cells with BSO caused a reduction in transfection efficiency of TETA-CBA. In addition, the extent of reduction in GLP-1 expression was much higher in the cells treated with the TETA-CBA formulation when IL-1β stimulation was given (Figure 5). However, PEI-mediated transfections were not affected by the BSO treatment. This result strongly supports our initial hypothesis in which the triggered release of the plasmid DNA in the cytoplasm could promote the chance of interaction between NFκB and the plasmid DNA. The interaction would lead to the improved nuclear translocation, resulting in enhanced expression of GLP-1.
Mimicking viral infection mechanism for improved transfection requires the elaborate integration of functional elements, including targeting moieties, endosomal escape, intracellular trafficking and nuclear translocation functionalities into a nano-scale container, i.e., a nanoparticle. In this study, we introduced a gene delivery system that can take advantage of endogenous nuclear transport mechanisms by combining a reducible polymeric gene carrier and a plasmid DNA containing repeated binding motif for a karyophilic protein, NFκB. The delivery system could successfully achieve enhanced transfection by efficient coordination of cytosolic triggered release and NFκB-mediated nuclear translocation mechanisms. This demonstrates an alternative strategy for integrating several functionalities in a polyelectrolyte-based nanoparticular container. Besides the intracellular delivery, additional functionalities, including improved extracellular stability and tissue specificity, are to be incorporated to produce a gene delivery system that eventually mimics virus infection machinery.
Figure 6.
Effect of DL-buthionine sulfoxamine (BSO) on transfection efficiencies of formulations based on either TETA-CBA or PEI. Activation and nuclear translocation of NFκB was initiated by the addition of IL-1β. The expression level of secreted GLP-1 was determined by ELISA and given as the mean ± S.D. (n=3). *p<0.01 compare to the PEI-based control.
ACKNOWLEDGMENTS
This work is supported by the grant from Korea Research Foundation (KRF-2008-331-D00756), the Ministry of Education, Science and Technology, Republic of Korea (2009-0059223, 2009-0093632, 2009-0088722), and US NIH (NIDDK-DK77703).
REFERENCES
- (1).Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–86. doi: 10.1016/j.addr.2006.03.007. [DOI] [PubMed] [Google Scholar]
- (2).Glover DJ, Lipps HJ, Jans DA. Towards safe, non-viral therapeutic gene expression in humans. Nat Rev Genet. 2005;6:299–310. doi: 10.1038/nrg1577. [DOI] [PubMed] [Google Scholar]
- (3).Schaffer DV, Fidelman NA, Dan N, Lauffenburger DA. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol Bioeng. 2000;67:598–606. doi: 10.1002/(sici)1097-0290(20000305)67:5<598::aid-bit10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- (4).Varga CM, Tedford NC, Thomas M, Klibanov AM, Griffith LG, Lauffenburger DA. Quantitative comparison of polyethylenimine formulations and adenoviral vectors in terms of intracellular gene delivery processes. Gene Ther. 2005;12:1023–32. doi: 10.1038/sj.gt.3302495. [DOI] [PubMed] [Google Scholar]
- (5).Tseng WC, Haselton FR, Giorgio TD. Mitosis enhances transgene expression of plasmid delivered by cationic liposomes. Biochim Biophys Acta. 1999;1445:53–64. doi: 10.1016/s0167-4781(99)00039-1. [DOI] [PubMed] [Google Scholar]
- (6).Branden LJ, Mohamed AJ, Smith CI. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat Biotechnol. 1999;17:784–7. doi: 10.1038/11726. [DOI] [PubMed] [Google Scholar]
- (7).Tanimoto M, Kamiya H, Minakawa N, Matsuda A, Harashima H. No enhancement of nuclear entry by direct conjugation of a nuclear localization signal peptide to linearized DNA. Bioconjug Chem. 2003;14:1197–202. doi: 10.1021/bc034075e. [DOI] [PubMed] [Google Scholar]
- (8).Mesika A, Kiss V, Brumfeld V, Ghosh G, Reich Z. Enhanced intracellular mobility and nuclear accumulation of DNA plasmids associated with a karyophilic protein. Hum Gene Ther. 2005;16:200–8. doi: 10.1089/hum.2005.16.200. [DOI] [PubMed] [Google Scholar]
- (9).Choi S, Oh S, Lee M, Kim SW. Glucagon-like peptide-1 plasmid construction and delivery for the treatment of type 2 diabetes. Mol Ther. 2005;12:885–91. doi: 10.1016/j.ymthe.2005.03.039. [DOI] [PubMed] [Google Scholar]
- (10).Goncalves C, Ardourel MY, Decoville M, Breuzard G, Midoux P, Hartmann B, Pichon C. An optimized extended DNA kappa B site that enhances plasmid DNA nuclear import and gene expression. J Gene Med. 2009;11:401–11. doi: 10.1002/jgm.1312. [DOI] [PubMed] [Google Scholar]
- (11).Christensen LV, Chang CW, Kim WJ, Kim SW, Zhong Z, Lin C, Engbersen JF, Feijen J. Reducible Poly(amido ethylenimine)s Designed for Triggered Intracellular Gene Delivery. Bioconjug Chem. 2006;17:1233–40. doi: 10.1021/bc0602026. [DOI] [PubMed] [Google Scholar]
- (12).Jeong JH, Christensen LV, Yockman JW, Zhong Z, Engbersen JFJ, Kim WJ, Feijen J, Kim SW. Reducible poly(amido ethylenimine) directed to enhance RNA interference. Biomaterials. 2007;28:1912–1917. doi: 10.1016/j.biomaterials.2006.12.019. [DOI] [PubMed] [Google Scholar]
- (13).Oh S, Lee M, Ko KS, Choi S, Kim SW. GLP-1 gene delivery for the treatment of type 2 diabetes. Mol Ther. 2003;7:478–83. doi: 10.1016/s1525-0016(03)00036-4. [DOI] [PubMed] [Google Scholar]
- (14).Jeong JH, Kim SW, Park TG. Molecular design of functional polymers for gene therapy. Progress in Polymer Science. 2007;32:1239–1274. [Google Scholar]
- (15).Luby-Phelps K, Castle PE, Taylor DL, Lanni F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc Natl Acad Sci U S A. 1987;84:4910–3. doi: 10.1073/pnas.84.14.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dowty ME, Williams P, Zhang G, Hagstrom JE, Wolff JA. Plasmid DNA entry into postmitotic nuclei of primary rat myotubes. Proc Natl Acad Sci U S A. 1995;92:4572–6. doi: 10.1073/pnas.92.10.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Carlotti F, Chapman R, Dower SK, Qwarnstrom EE. Activation of nuclear factor kappaB in single living cells. Dependence of nuclear translocation and anti-apoptotic function on EGFPRELA concentration. J Biol Chem. 1999;274:37941–9. doi: 10.1074/jbc.274.53.37941. [DOI] [PubMed] [Google Scholar]
- (18).Ding GJ, Fischer PA, Boltz RC, Schmidt JA, Colaianne JJ, Gough A, Rubin RA, Miller DK. Characterization and quantitation of NF-kappaB nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J Biol Chem. 1998;273:28897–905. doi: 10.1074/jbc.273.44.28897. [DOI] [PubMed] [Google Scholar]
- (19).Mesika A, Grigoreva I, Zohar M, Reich Z. A regulated, NFkappaB-assisted import of plasmid DNA into mammalian cell nuclei. Mol Ther. 2001;3:653–7. doi: 10.1006/mthe.2001.0312. [DOI] [PubMed] [Google Scholar]
- (20).Christensen LV, Chang CW, Yockman JW, Conners R, Jackson H, Zhong Z, Feijen J, Bull DA, Kim SW. Reducible poly(amido ethylenediamine) for hypoxia-inducible VEGF delivery. J Control Release. 2007;118:254–61. doi: 10.1016/j.jconrel.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lee Y, Mo H, Koo H, Park JY, Cho MY, Jin GW, Park JS. Visualization of the degradation of a disulfide polymer, linear poly(ethylenimine sulfide), for gene delivery. Bioconjug Chem. 2007;18:13–8. doi: 10.1021/bc060113t. [DOI] [PubMed] [Google Scholar]
- (22).Kim SH, Jeong JH, Kim TI, Kim SW, Bull DA. VEGF siRNA delivery system using arginine-grafted bioreducible poly(disulfide amine) Mol Pharm. 2009;6:718–26. doi: 10.1021/mp800161e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Read ML, Singh S, Ahmed Z, Stevenson M, Briggs SS, Oupicky D, Barrett LB, Spice R, Kendall M, Berry M, Preece JA, Logan A, Seymour LW. A versatile reducible polycation-based system for efficient delivery of a broad range of nucleic acids. Nucleic Acids Res. 2005;33:e86. doi: 10.1093/nar/gni085. [DOI] [PMC free article] [PubMed] [Google Scholar]