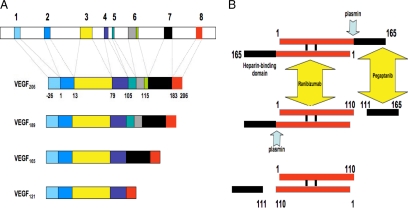
Figure 1.
(A) Organization of the human VEGF-A gene. The gene comprises of eight exons. Alternative splicing results in the generation of multiple isoforms (Houck et al., 1991; Tischer et al., 1991). The monomeric precursor of each isoform is represented here. The most frequently detected isoforms are VEGF121, VEGF165, and VEGF189. Less abundant splice variants have been described, including VEGF145, VEGF162, VEGF183, and VEGF165b. VEGF165 interacts with HSPG through exon 7–encoded sequences. VEGF189 and VEGF206 have one additional heparin-binding domain, encoded by exon 6, explaining the particularly strong binding of these isoforms to HSPG in the ECM. (B) Plasmin-mediated cleavage of VEGF165. The mature VEGF homodimer is represented here. Note the sequential cleavage, generating first the heterodimer VEGF165/110 (Keyt et al., 1996). The final product is VEGF110, lacking the sequences encoded by exons 7 and 8 and part of exon 5. This protein is biologically and biochemically similar to alternatively spliced VEGF121. The diagram also illustrates the site-specific binding of two inhibitors, ranibizumab and pegaptanib. VEGF110 activity is blocked by ranibizumab but not by pegaptanib.