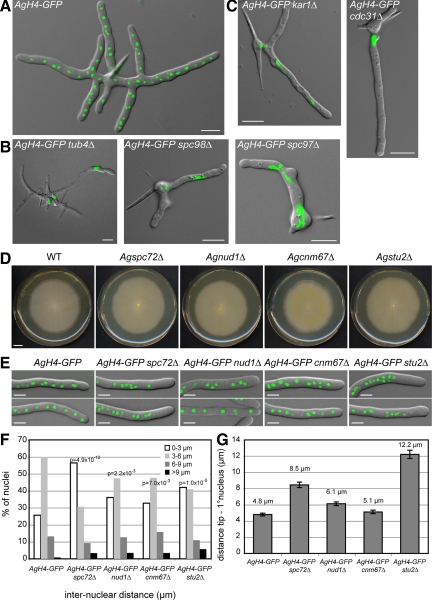
Figure 1.
Deletion of A. gossypii SPB components. Overlays of DIC image and AgH4-GFP signals from cells grown for ∼12 h at 30°C. (A) A typical wild-type mycelium at this stage contains multiple branches and 50–100 nuclei. (B) Terminal phenotype of mutants lacking components of the γ-tubulin complex. (C) Terminal phenotype of mutants lacking half-bridge components. Some mutants shown in B and C arrest as small mycelium with up to 12 branches containing a few nuclei and did not arrest as germlings with one nucleus or two nuclei. This is due to a maternal effect, where remnants of the wild-type protein are packed into mutant spores (Gladfelter et al., 2006). In A. gossypii, targeted deletions are performed with young mycelium containing ∼20 haploid nuclei that initially generate heterokaryotic strains: mixtures of nuclei carrying either the wild type or the deletion allele. On starvation, haploid mononucleate spores are produced in the heterokaryotic hyphae, and a substantial part of the mutant spores contain wild-type (maternal) protein, which can account for early growth and nuclear division in otherwise fatal deletions. Bars, 10 μm. (D) Radial growth of wild-type and deletion mutants on solid medium during 7 d of incubation at 30°C. Bar, 1 cm. (E) Overlays of DIC image and AgH4-GFP signals from hyphae showing nuclear distributions in wild-type and mutant strains. Bars, 5 μm. (F) Distribution of distances between adjacent nuclei was plotted using distance measurements between the nuclei in the first 50 μm of at least 20 different hyphae for each strain (n > 200 for each strain). The center of the GFP signal was used as the central point of the nucleus. Confidence values (p) for the χ2 test were calculated for each data set between wild-type and mutant distributions. (G) Distance between the hyphal tip and the first nucleus was determined. Error bars represent the SE of the mean, n > 60 for each strain.