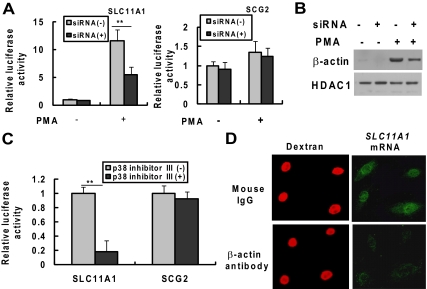
Figure 8.
Nuclear β-actin is required for PMA-induced transactivation of SLC11A1. HL-60 cells were respectively transfected with siRNA control or siRNAs specific for β-actin. Twenty-four hours after transfection, cells were transiently cotransfected again with the luciferase reporter vector pREP4-SLC11A1-Luc or pREP4-SCG2-Luc with pRL-CMV. Transfected cells were cultured for 48 h with or without PMA (10 ng/ml). (A) Total protein extracts were prepared and the luciferase activities driven by SLC11A1 or SCG promoter were analyzed by the dual-luciferase reporter assay system. (B) Nuclear extracts were prepared and β-actin and HDAC1 expression was detected by Western blot analysis. (C) HL-60 cells were transiently cotransfected with the luciferase reporter vector pREP4-SLC11A1-Luc or pREP4-SCG2-Luc with pRL-CMV. The cells were treated with the p38 MAPK inhibitor (p38 inhibitor III; 20 μM) at 24 h posttransfection and then treated with PMA (10 ng/ml) 1 h later. The luciferase activities were analyzed at 48 h after PMA treatment. The data shown (mean ± SE) are the averages of three independent experiments performed in triplicate. **p < 0.001, compared with the respective group of untreated cells. (D) Microinjection of antibody to β-actin inhibits SLC11A1 transcription in vivo. HL-60 cells were treated with PMA (10 ng/ml) for 30 h and were then microinjected into the nuclei with an antibody against β-actin or with mouse IgG as a control, together with TRITC-labeled dextran to identify the injected cells. Eighteen hours after microinjection, the cells were fixed with 3.7% formaldehyde and hybridized in situ with biotinylated anti-sense RNA probe to SLC11A11 mRNA. The biotinylated probe was developed for visualization with alkaline phosphatase-mediated techniques using the ELF97 mRNA in situ hybridization kit. Cells containing the biotinylated probes were then detected using streptavidin-alkaline phosphatase conjugate and the ELF97 phosphatase substrate.