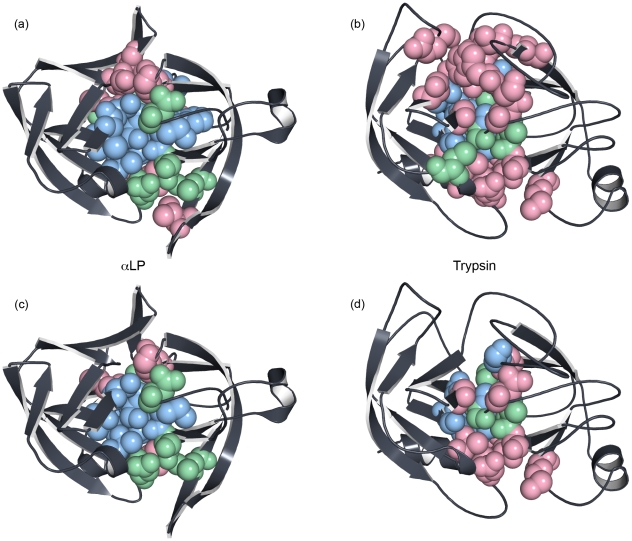
Figure 9. Solvation of the domain interface during unfolding differs significantly between αLP and trypsin.
(a) - (d) Residues colored light red are solvent-exposed at the TSE, light green residues become exposed within 600 ps of the TSE, and light blue residues are still buried 600 ps past the TSE. (a) and (c) αLP, (b) and (d) trypsin. (a) and (b) All buried domain interface residues, (c) and (d) the subset of (a) and (b) where the position is conserved and found at the domain interfaces of both prokaryotic and metazoan proteases. Notably, many fewer buried residues of the αLP domain interface are solvated at the TSE compared to trypsin, even after eliminating the non-conserved positions.