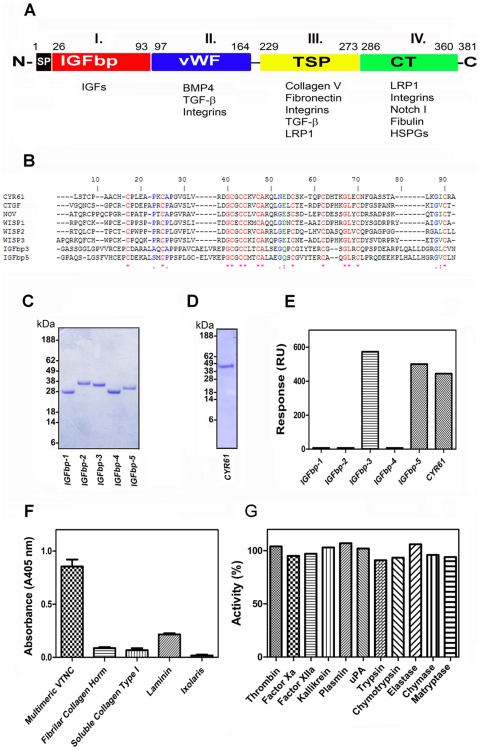
Figure 1. Cyr61 binds to VTNC.
(A) Structure of CCN family members. CCN1 (Cyr61, represented in the figure), CCN2 (CTGF), CCN3 (NOV), CCN4 (WISP-1), CCN5 (WISP-2), and CCN6 (WISP-3) have a shared structure consisting of a secretory signal peptide (SP), an IGFBP domain (Module I), a von Willebrand type C domain (vWC, Module II), a thrombospondin-1 domain (TSP, Module III) and a cysteine knot (CT, Module IV) domain. Domains are linked by hinge regions susceptible to protease cleavage. Modified from reference [3]. (B) Clustal alignment of CCN family members with IGFBP-3 and -5 indicates several conserved residues (*). (C) and (D) SDS-PAGE for IGFBP-1 to -5, and Cyr61, respectively. Two µg of proteins were loaded in a 4–12% NU-PAGE gel, which was Coomassie blue stained. (E) SPR experiments: proteins (100 nM) were used as analytes for immobilized VTNC. Binding levels at stability was used as a value for comparison. RU, resonance units. (F) Specificity of Cyr61 (30 nM) tested by solid-phase binding assays: proteins were immobilized as the following densities/well: VTNC (25 ng), fibrilar collagen (50 ng), ixolaris (75 ng), soluble collagen and laminin (100 ng), followed by incubation with Cyr61 (30 nM). Bound-Cyr61 was identified using anti-Cyr61 monoclonal antibody. (G) Cyr61 does not inhibit enzyme activity. Cyr61 (300 nM) was incubated with different enzymes and fluorogenic substrate hydrolysis was followed as indicated in Materials and Methods. SEM are not depicted but were less than 5% (n = 3).